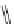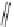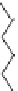Biomedical Engineering Reference
In-Depth Information
O
-
O
N
10, Diphenylamine carboxylate
HO
OH
HO
-
O
O
HO
OH
11, Gluconate
O
H
N
H
N
S
O
Cl
O
O
H
O
12, Glibenclamide
O
HO
13, Arachidonic acid
cytoplasmic mouth of the pore, where it can attract the Cl
into the pore by
a surface charge mechanism [
177
]. Thus, arachidonic acid is thought to enter the
cytoplasmic end of the channel pore to inhibit the CFTR Cl
currents, most likely
by physically occluding the Cl
permeation pathway [
176
]. According to Linsdell
[
174
], however, other negatively charged substances, too, such as sulfonyl ureas,
disulfonic stilbenes, indazoles, arylaminobenzoates, and conjugated bile salts,
inhibit CFTR Cl
currents by interacting electrostatically with K95 and blocking
the open channel. However, while the inhibition by these substances is weakened
by the depolarization of the membrane potential [
169
,
174
] and increase in extra-
cellular Cl
concentration, the arachidonic inhibition is practically independent of
both membrane potential and Cl
concentration [
175
].