Biomedical Engineering Reference
In-Depth Information
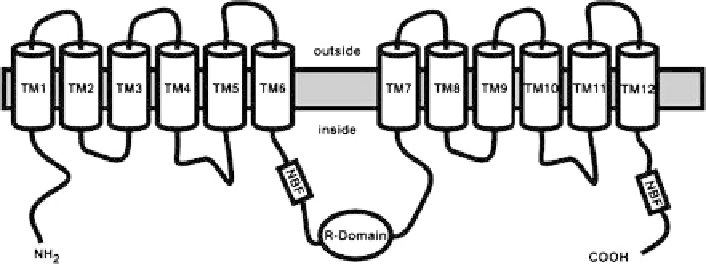
Fig. 2 A schematic representation of cystic fibrosis transmembrane conductance regulator
(CFTR) that has 12 transmembranes (TMs), two nucleotide binding folds (NBFs), and a regulatory
R domain. Reprinted with permission from [
9
]. Copyright 2002 the American Physiological
Society
are found in many cell types, for example, epithelial cells [
64
,
65
], neurons [
66
-
68
],
cardiac cells [
69
], smooth muscle cells [
70
], and blood cells [
71
-
73
]. An important
role of these channels is played in epithelial cells where they act as transepithelial
transporter [
74
].
Some Cl
channels are activated by extracellular calcium ions, for example,
cloned renal Cl
channels [
75
,
76
] and certain of those found in
Xenopus
oocytes [
77
]. In most of the cases the activation of Cl
channels by Ca
2+
involves the phosphorylation by Ca
2+
/calmudulin-dependent protein kinase II,
for example, in human T84 and H-29 colonic cells [
78
,
79
], in normal and cystic
fibrosis airway epithelial cells [
73
], and in
Xenopus laevis
oocytes [
80
]. How-
ever, in certain cases the phosphorylation was not found to be involved in the
activation of Cl
channels by Ca
2+
, for example, in parotid secretory cells [
78
]
and rat submandibular acinar cells [
81
]. In addition, however, the phosphoryla-
tion by the Ca
2+
/calmodulin-dependent protein kinase II may also be involved in
inactivation of Cl
channels by Ca
2+
as observed by Wang and Kotlikoff [
82
]in
smooth muscle.
2.4 Ligand-Gated Cl
Channels
The GABA and glycine (Gly) receptors that belong to the family of LGICs act as
Cl
channels. The LGICs, also known as ionotropic receptors, open in response to
specific ligand molecules binding to extracellular domain of the receptor protein.
Ligand binding causes a conformational change in the structure of the channel
protein that ultimately leads to the opening of the channel gate and subsequent ion
flux across the plasma membrane.
The LGICs are pentameric proteins, in which each subunit has a large extra-
cellular domain at
the N-end, four transmembrane (TM) segments, and an