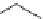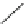Biomedical Engineering Reference
In-Depth Information
Fig. 3 Fragments found in
hERG blockers. The asterisk
indicates the attachment point
*
H
N
A
*
N
O
A
O
NN
Fragment A
Fragment B
be more complex. The possibility to reduce the hERG affinity increases if the
transformation is adjacent to an aliphatic chain. The opposite effect is obtained if
the transformation is next to a hydrogen-bond acceptor in an aromatic ring. On the
contrary, if the transformation is adjacent to an aromatic ring the hERG inhibition is
not affected. These results are related to the possibility to form hydrogen-bonds by
the oxygen atom of the methoxy group. In the case of the substitution of a methyl
group with a fluorine atom, the global distribution indicates that this might result in
an increase or a decrease of the hERG affinity. The presence of the fragment A
(Fig.
3
) is correlated with the increase of hERG potency. The analysis of the global
distribution shows that replacing the cyclohexyl with the phenyl group can reduce
or improve hERG potency. The cyclohexyl
phenyl transformation in the
fragment B (Fig.
3
) increases the probability to obtain a more potent hERG blocker.
This result is due to the increased lipophilicity and hydrophobicity of the molecule,
and to the reduced ability to form hydrogen-bonds of the adjacent amidic carbonyl.
These three examples show the importance to include contextual information in the
drug discovery process to develop compounds with a good toxicological profile.
>>
5 Structure-Based Approaches
5.1 Homology Models of the hERG Channel
Up to date there is no crystal structure of the hERG channel and most of our
knowledge comes from studies on the Shaker channel (Kv1.1) and on mammalian
channels (Kv1.2), KcsA, MthK, and KvAP. Since the eukaryotic and prokaryotic
pores are closely related, it appears reasonable to use the crystal structures of KcsA
(pdb codes 1BL8 [
79
], 1K4C [
80
] and 1R3J [
81
]) and of KirBac1.1 (1P7B [
82
]) to
build homology models of the hERG channel in the closed state, or MthK (1LNQ
[
83
]) and KvaP (1ORQ [
84
]) for the open state. It has to be noted that the degree of
the pore opening varies in the crystal structures, from the closed state (KcsA) to the
open state (KvaP), up to an even more open state in MthK. These different degrees
in pore opening might represent different gating properties of the K
þ
channel, or
different snapshots in the gating trajectory. The first homology models of hERG
channel were discussed also in several reviews [
26
,
27
,
85
-
87
].
Many groups modeled the hERG channel in the closed state using as template
the crystal structures of KcsA (1BL8 or 1K4C) [
4
-
6
,
11
,
12
,
88
-
94
], as well as of
MthK (1LNQ) [
11
,
21
,
49
,
88
,
89
,
94
]. The homology models of the hERG channel