Biomedical Engineering Reference
In-Depth Information
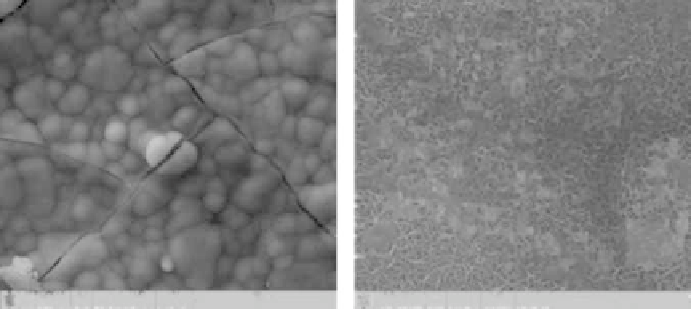
FIGURE 2.4
Apatite mineralization on silicate bioceramics with lath-like morphology in SBF.
ability was directly dependent on their chemical compositions and disso-
lution. Wollastonite, dicalcium silicate, tricalcium silicate, bredigite, and
nagelschmidtite ceramics possess the best apatite-mineralization ability
and quickest dissolution in SBF. Akermanite, merwinite, silicocarnotite, and
strontium silicate have good apatite mineralization and moderate dissolu-
tion. Hardystonite bioceramics have no obvious apatite mineralization and
their dissolution is quite low. The morphology of the formed apatite on typi-
cal silicate ceramics is shown in Figure 2.4. Generally, silicate bioceramics
with high Ca contents possess improved apatite mineralization in SBF. The
incorporation of other metal ions, such as Mg, Zn, and Zr, into binary oxide
silicate ceramics will decrease their apatite mineralization. Furthermore, it
is found that the dissolution may play an important role to influence the
apatite mineralization of silicate ceramics. Silicate ceramics with quick dis-
solution possess improved apatite mineralization ability, compared to those
with slow dissolution. Our studies have shown that the apatite formation
of silicate bioceramics may involve several steps. First, the ion exchange of
Ca
2+
and other metal ions in the silicate ceramics with H
+
in SBF resulted in
the formation of a hydrated silica layer on the surfaces of the ceramics and
provided favorable sites for phosphate nucleation (Wu, Chang, Wang, et al.
2005). Then, Ca
2+
and PO
4
3-
ions in SBF will remineralize and form amor-
phous calcium phosphate and the subsequent formation of crystal apatite
by incorporating OH
-
ions from SBF. The mechanism of apatite formation
on the surfaces of silicate bioceramics is similar to that of 45S5 bioglass (Wu,
Chang, Wang, et al. 2005; Wu and Chang, forthcoming).
Besides the apatite-mineralization properties of silicate bioceramics, it was
found that some silicate ceramics, such as dicalcium silicate and tricalcium
silicate, possessed a self-setting property and injectability. The self-setting
property of silicate paste is due to the progressive hydration of the SiO
4
4−
ions
in silicate ceramics. When silicate ceramics react with water, a nanoporous,

Search WWH ::

Custom Search