Biomedical Engineering Reference
In-Depth Information
Ca
2+
-H
2
PO
4
-Urea
Crystalline nucleation
Dissolution and
recrystallization
Self-assembly
Further self-assembly
and �ower formation
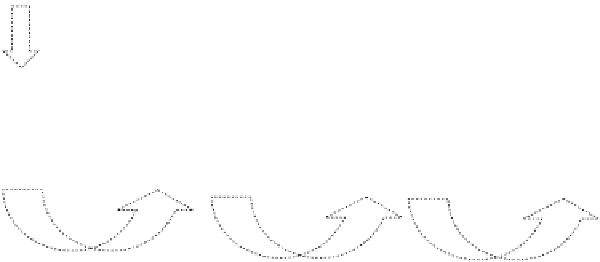
FIGURE 6.5
Schematic illustration of the formation and morphology evolution of 3D structured HAp flow-
ers in the whole synthetic process.
Mg(NO
3
)
2
, KNO
3
, NH
4
Cl, and NH
4
F were used as substituted ion sources;
and urea was applied as the homogeneous precipitation reagent and CO
3
2-
source. The characterization results showed that the obtained biomimetic
HAp porous microspheres were self-assembled by 2D single crystalline
nanosheets. The novel 3D architectures resulted in favorable drug load-
ing and release properties, and the cosubstituted essential trace elements
decreased the crystallinity of the products and enhanced the degradability
of the porous microspheres in comparison with the traditional pure HAp
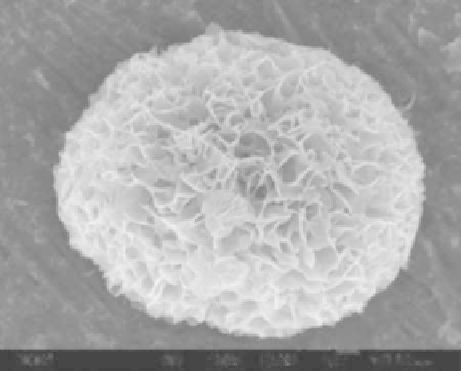
FIGURE 6.6
FESEM image of the synthetic HAp porous microspheres with cosubstituted essential trace
elements (Na, Mg, K, F, Cl, and CO
3
2-
) of natural bone.



Search WWH ::

Custom Search