Biomedical Engineering Reference
In-Depth Information
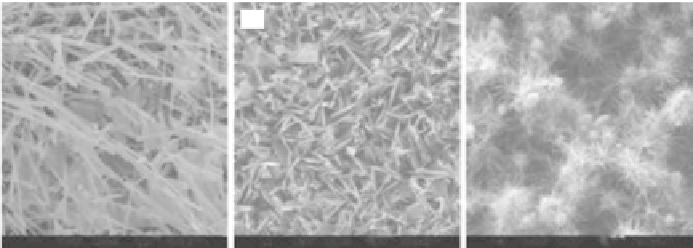
(c)
(a)
(b)
FIGURE 6.2
FESEM images of HAp powders with different morphologies: (a) rodlike, (b) platelike, (c)
chrysanthemum-like.
HAp microrods) could be obtained (Figure 6.2) (Liu et al. 2011). Recently,
we developed a novel process to synthesize element-substituted HAp with
controllable morphologies and chemical compositions using calcium silicate
as hard-template precursors in the absence of any surfactants and additives
(Lin, Chang, Liu, et al. 2011). When hydrothermal treatment of the amor-
phous calcium silicate hydrate (CSH) precursor in Na
3
PO
4
solution occurs,
HAp nanoparticles with diameters about 90 nm were obtained (Figure 6.3a).
In contrast, when the crystalline calcium silicate (CS) powders were used as
the precursor and the Na
3
PO
4
solution was used as the phosphorus source,
the obtained HAp powders consisted of nanowires with lengths up to 2 µm
and diameters about 100 nm (Figure 6.3b). Figure 6.3c illuminates that the
obtained HAp powders via hydrothermal treatment with the crystalline CS
powders in NaH
2
PO
4
solutions had a smooth surface and ultralong sheetlike
shape with thickness about 100 nm, widths 1~5 µm, and lengths up to 20 µm.
The model of Ca
9
(PO
4
)
6
clusters (Posner clusters) with positive charge could be
(c)
(a)
(b)
FIGURE 6.3
FESEM images of HAp powders with different morphologies: (a) nanoparticles (CSH as pre-
cursor, Na
3
PO
4
as solution); (b) nanowires (CS as precursor, Na
3
PO
4
as solution), (c) nanosheets
(CS as precursor, NaH
2
PO
4
as solution).







Search WWH ::

Custom Search