Biomedical Engineering Reference
In-Depth Information
2.4 Monitoring of Cellular Response to Recombinant
Gene Expression
2.4.1 Nucleotides
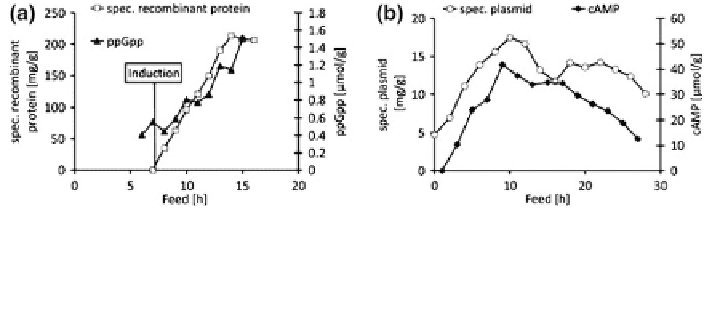
Monitoring of nucleotides such as guanosine tetraphosphate (ppGpp), the central
molecule of the stringent response system, and cyclic adenosine monophosphate
(cAMP), a signal molecule involved in metabolite repression, is of great interest.
Hence, a method for quantification of these molecules was developed, providing
the ppGpp stress response to recombinant gene expression [
51
]. Monitoring of
concentration levels of these molecules yields valuable information on cellular
state and product formation. As shown in Fig.
2
a, recombinant protein is accu-
mulated in the cells after induction for approximately 7 h. After this period, the
metabolism became overloaded and cells were no longer able to maintain growth
and product formation. The level of ppGpp shows a similar course, with a sig-
nificant increase after induction clearly indicating the high stress level in the cells.
In Fig.
2
b, cAMP and quantitative plasmid data from a plasmid production process
are shown. The course of cAMP is very similar to the trend of specific plasmid
content in the cells [
52
]. This finding strengthens the hypothesis that plasmids
affect host metabolism through the perturbation of the cAMP-CRP complex, as
postulated by Ricci et al. [
29
].
2.4.2 Proteome and Transcriptome Analysis
Since recombinant gene expression affects the host cell metabolism on a genome-
wide scale, it is advisable to extend the investigations on this level. In terms of a
systems-biology-based approach, changes to the transcriptome and proteome
pattern can provide host and recombinant protein-specific information and valu-
able clues for metabolic engineering targets to cope with physiological bottlenecks
of the host metabolism.
Fig. 2 a Courses of ppGpp and specific recombinant protein per gram cell dry mass in carbon-
limited exponential fed-batch cultivation (growth rate 0.1 h
-1
)ofE. coli HMS174(DE3)(pET30a-
SOD). b Courses of cAMP and specific content of plasmid per gram cell dry mass in carbon-limited
exponential fed-batch cultivation (growth rate 0.1 h
-1
)ofE. coli JM108(pMCP-1)
Search WWH ::

Custom Search