Biomedical Engineering Reference
In-Depth Information
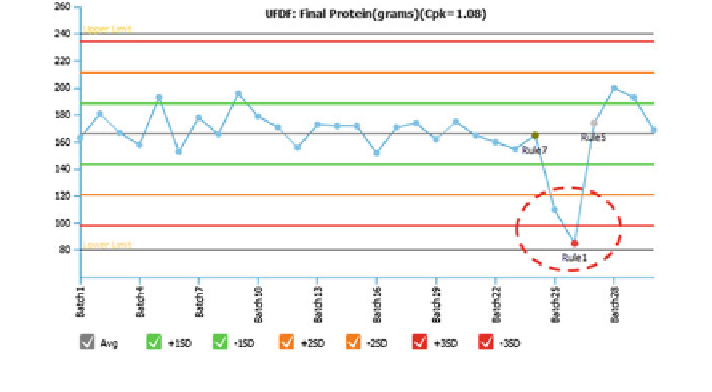
Fig. 17
Statistical process control chart for a downstream process parameter
4 Case Studies
Any cGMP campaign involves resolution of deviations and non-conformances that
occur during process execution. Resolution of these process issues requires a
detailed root cause investigation so that effective corrective actions can be taken to
prevent
future
occurrences.
A
typical
root
cause
investigation
starts
with
answering some of the following questions:
• What was the observation?
• At what stage (process step) was it observed in the process?
• Was any other abnormal behaviour observed for this process step?
• Was everything OK with raw materials or intermediate products that feed this
process step?
• Does a similar trend or observation exist in earlier steps that feed the current
process step?
An efficient process monitoring and knowledge management program (as dis-
cussed in earlier sections) will equip the organization with the information
required to answer these questions. The cases discussed next detail the investi-
gative procedure usually followed for a root cause investigation or process
improvements.
Search WWH ::

Custom Search