Biomedical Engineering Reference
In-Depth Information
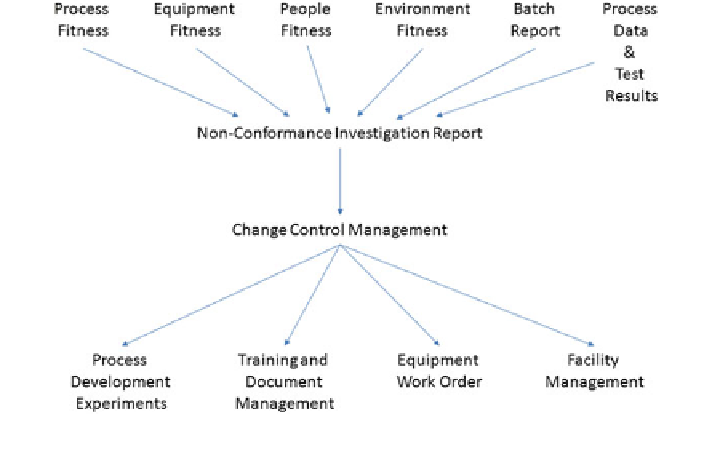
Fig. 6
Flow of information during the root cause investigation process
Figures
5
and
6
shows the lifecycle of various data, information and knowledge
repositories used in bioprocess manufacturing. An open and unified data man-
agement approach across all functional groups involved in process execution
within an organization will significantly help in efficient bioprocess knowledge
management.
2 Process Monitoring
2.1 Introduction
Process monitoring can be defined as an approach involving collection of process
data and statistical evaluation of parameters to verify or demonstrate that the
process is operating in a state of control, identify possible process changes and
shifts and promote continuous improvement. Consistency in product quality each
time the batch is executed requires maintaining process variables within the
specified limits for each batch. In pharmaceutical manufacturing, where the pro-
cesses are complex and have interdependent multistage operations, each of which
has numerous variables that affect the process, achieving consistent quality
becomes a challenging goal. This makes it even more important to monitor these
variables from batch to batch.
Generally, the process monitoring program starts with identifying the param-
eters relevant to the process and then recording the behaviour of those parameters
Search WWH ::

Custom Search