Biomedical Engineering Reference
In-Depth Information
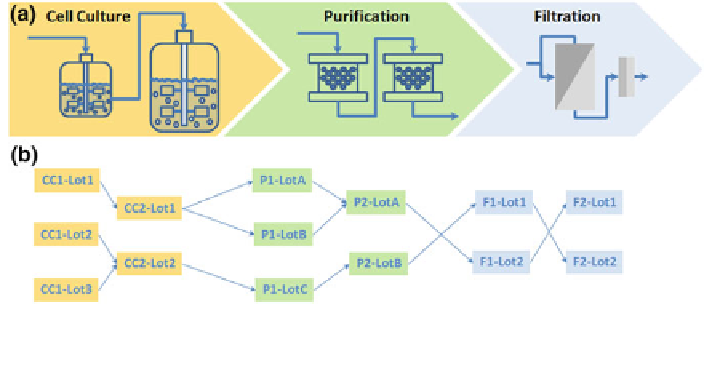
Fig. 4 a Example execution sequence of bioprocess unit operations. b Lot genealogy, involving
multiple splits and combinations
operation. Due to biological uncertainty and inherent process variability, the
upstream titres at the cell culture stage dictate the overall process sequence and
hence the need for flexible downstream processing. This flexibility usually results
in various splits, combinations and process step cycles, as shown in Fig.
4
b, and
poses a significant challenge in data management and root cause resolution.
1.4 Data Flow Map
Biomanufacturing involves leveraging a large amount of information for suc-
cessful process execution and continuous improvement. This information set
consists of not only process data from the current batch/run but also data from
historical batches and execution-related information, including:
• Data from process development runs and technology transfer reports.
• Previously executed commercial batches.
• Equipment usage and maintenance records.
• Clean-room environment data.
• Personnel training data.
• Current and historical process non-conformances and deviations.
Figure
5
shows various data, information and knowledge repositories and how
the content flows from one to another during normal execution of the commercial
manufacturing process lifecycle of a product batch. The information is maintained
in systems owned by various functional groups such as process development,
engineering, quality control, quality assurance and manufacturing.
As shown in Fig.
5
, process development engineers and scientists conduct
experiments to understand and develop a robust production process for manu-
facturing. The outcome of these experiments is development and technology
transfer reports. These reports form the basis of process validation activities for
Search WWH ::

Custom Search