Biomedical Engineering Reference
In-Depth Information
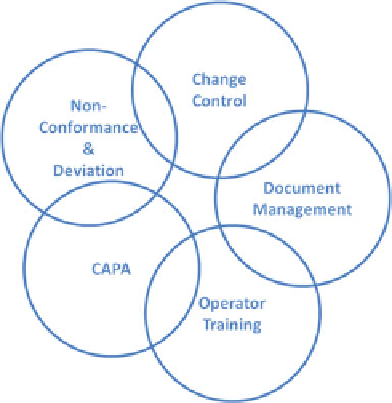
Fig. 2 Elements of a quality
management system
• Quality management systems (QMS): Records of controlled documents such as
BPRs, standard operating procedures (SOP), non-conformance investigation
reports and operator training records are all maintained for the full lifecycle of
the product. The main process execution compliance systems are interlinked to
each other as shown in Fig.
2
and are an integral part of the complete knowledge
infrastructure. These carry useful information including batch reports, non-
conformances, investigational and technical reports that contain vital process
knowledge that is important for continuous improvement initiatives.
1.3.2 Data Capture Modes
Bioprocess execution data are typically captured in three modes: offline, at-line,
and online. These modes are defined by the data sampling frequency, the time it
takes to obtain the results and the distance the test sample has to travel. The modes
are explained as follows:
• Offline data capture: Offline data comprise mainly test results on samples taken
during processing that need to be sent for testing to a location outside of the
processing room. Typically these data are captured and maintained by quality
control laboratories within an organization or off-site testing agencies. The data
are recorded in paper records, spreadsheets, custom databases or LIMS systems.
• At-line data capture: At-line data are manual data captured near the process
equipment on the processing floor, being recorded in paper records, spread-
sheets, custom databases or electronic batch records (e.g. cell count data,
metabolite data, processing times etc.). These data are typically recorded by
scientists or operators executing the process batch.
Search WWH ::

Custom Search