Agriculture Reference
In-Depth Information
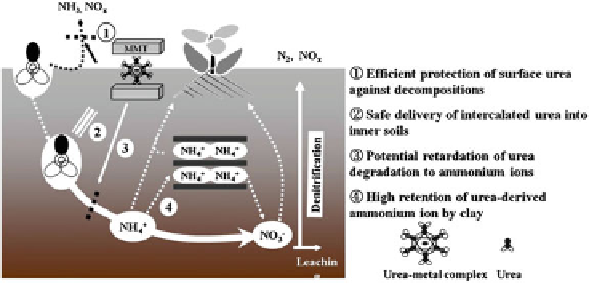
Fig. 11.3 An illustration for behaviors of intercalated urea molecules in soils. Reprinted (adapted)
with permission from Kim et al. (
2011
), copyright 2014 Springer
material from an agronomic point of view, i.e., the material (prepared by physical
mixing of the constituents and melting at 105
C) was not studied in terms of final
properties and their relations with the processing method. It is noteworthy that the
agronomic data are motivating and allow one to suppose that more attention in the
composite production stages could maximize the final results.
Pereira et al. (
2012
) demonstrated that nanocomposites produced from mont-
morillonite exfoliation into urea matrix can control the solubilization process of
urea, delaying its release to environment. The results showed that it is possible to
obtain a material processable by cold extrusion, with high N content and strength
compatible to the final application. Microstructural analysis of the nanocomposites
showed that the extrusion process generated two regions, one comprising the
nanocomposite itself (montmorillonite and urea) and the other with urea granules.
Thus, the authors attributed the release process not only to the clay mineral-urea
interaction but also to the creation of barriers to free urea diffusion out of the
granules. Scanning electron microscopy (SEM) images of these nanocomposites
and a scheme of the proposed pathway for urea intercalation during extrusion
process are depicted in Fig.
11.4
.
Hydrogels have been studied as polymer matrixes for controlled-release appli-
cation, specially because of their interesting properties in the release of water-
soluble inputs. Hydrogels are materials formed by long, flexible polymer chains,
which can be linked by covalent (chemical or cross-linked hydrogels) or physical
interaction (physical hydrogels) (Peppas et al.
2000
). Under specific conditions,
these materials can absorb large quantities of water as well as a nutrient solution
containing some active compound (Campese et al.
2007
). The difference between
chemical and physical hydrogels is in their chain formation: chemical hydrogels
cannot be dissolved once they are formed, whereas physical hydrogels can be
dissolved with external stimuli such as changes in temperature, pH, ionic strength,
etc. Due to these properties, hydrogels are used in a wide range of sectors, strength
in the manufacture of hygiene products (Singh et al.
2010
), agriculture (Leone
et al.
2008
; Sorbara et al.
2009
; Zhang et al.
2009
), drug delivery (Hamidi