Agriculture Reference
In-Depth Information
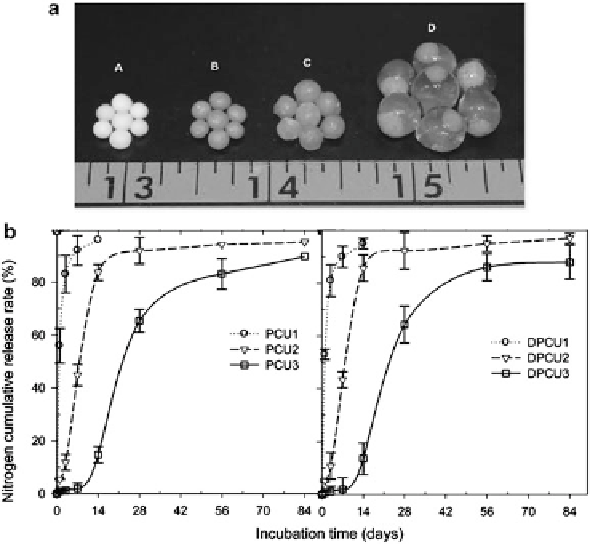
Fig. 11.2 (a) Images of fertilizer materials used in the experiments: urea (A), polymer-coated
urea (B), dry double-layer polymer-coated urea (DPCU) (B), and swollen DPCU (D); (b) cumu-
lative nitrogen release rate of different coated fertilizers at 25
C in water (A) and soil (B) to
double-layer polymer-coated urea (DPCU). Reprinted (adapted) with permission from Yang
et al. (
2013
), copyright 2014 American Chemical Society
coating, and poly(butyl methacrylate) as the outer coating. Costa et al. (
2013
)
studied urea granules coated with polyhydroxybutyrate and methyl cellulose
under various conditions, in the presence of emulsifiers, showing that the tested
polymers were effective as coating, leading to a reduction of the dissolution rate of
urea in water.
Nitrogen losses can also be reduced using zeolite as an additive to fertilizers in
order to control the retention and release of NH
4
+
. Zeolites are hydrated crystalline
aluminum silicate minerals of alkali or alkaline earth metals. They are structured in
rigid three-dimensional crystalline lattices formed by SiO
4
and AlO
4
tetrahedrons,
whose union of rings forms a system of channels, cavities, and pores (Ming and
Mumpton
1989
). This structural description is fairly comprehensive, allowing the
formation of zeolite structures with channels (mesopores) of around 3
to 2 nm.
Zeolites are naturally found in various forms (Ming and Mumpton
1989
), but they
can be easily synthesized with the same structures from aluminosilicate sources as
well (Mignoni et al.
2008
). Due to this mesoporous structure, zeolites generally
have high surface area (above 100 m
2
g
1
). However, the aluminosilicate structure
Å