Biomedical Engineering Reference
In-Depth Information
with guided by a systematic database amassed from nature but also human technology
through patents. Lastly, we study nature and try to faithfully copy the vital components
and reinvent this in the laboratory.
SBF Hydroxyapatite
Artificial materials implanted into bone defects might be encapsulated from time to time
by fibrous tissue, leading to their isolation from the surrounding bone. Improvement of
bone implant bonding to the tissues requires the right implant surface morphology and
chemistry to generate mechanical interlock and good surface activity. Interactions between
the bone and the implant will be controlled with appropriate biological interactions.
In the past three decades a large number of investigators have proposed that the essential
requirement for an artificial material to bond to living bone is the formation of bonelike
apatite on its surface when implanted in the living body. In 1991, Kokubo et al. (1992) pro-
posed that in vivo apatite formation on the surfaces of many biomedical materials can be
reproduced in SBF with ion concentrations nearly equal to those of human blood plasma.
In essence, this means that the in vivo bone bioactivity of a material can be predicted from
the apatite formation on its surface in SBF.
Hydroxyapatite layers can be easily produced on various organic and inorganic sub-
strates when submerged in simulated body fluid. In 1989, Kokubo and Takadama (2006)
showed that after immersion in SBF, a wide range of biomaterial surfaces initiated very
fine crystallites of carbonate ion-containing apatite, and since then a large number of stud-
ies have shown that osteoblasts can proliferate and differentiate on this apatite layer.
Since then, SBFs have been produced in order to provide insight into the reactivity of the
inorganic component of blood plasma, and predict the bioactivity of implants and bone
scaffolds, as well as other novel biomaterials. Kokubo et al. in a number of follow-up stud-
ies (Kokubo et al. 2000; Kokubo and Takadama 2006) warned that physiological environ-
ments contain not only the inorganic components but organics and dissolved gases and
investigators should be careful with the results obtained within the SBF.
SBF solutions are shown to induce apatitic calcium phosphate formation on metals,
ceramics, or polymers soaked in them. SBF solutions, in close resemblance to the Hanks'
balanced salt solution (HBSS) are prepared with the aim of simulating the ion concentra-
tions present in human plasma. To mimic human plasma, SBF solutions are prepared to
have relatively low calcium and phosphate ion concentrations, namely, 2.5 and 1.0 mM,
respectively. Furthermore, to mimic human plasma, the pH value of SBF solutions was
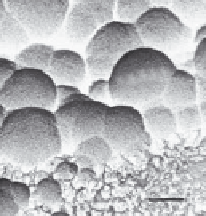
Apatite
3 µm
FIGURE 2.4
SBF coating on a porous surface (Kokubo et al. 2000).
Search WWH ::

Custom Search