Biomedical Engineering Reference
In-Depth Information
Epitaxial film
Platelike crystal
Whisker
Dendrite
Columnar grain
Fine crystal
Amorphous
Powder
FIGURE 7.16
Effects of temperature and supersaturation on morphology of deposits in CVD.
Conventional CVD is often termed thermal CVD because CVD is a sequential thermally
activated process including many chemical reactions and mass-transfer steps as illustrated
in Figure 7.18.
(53)
Therefore, CVD commonly requires a higher deposition temperature than
PVD; this may be a disadvantage of CVD. This disadvantage relates to the microstructural
change in Ti substrates during CVD processes. Ti transforms from α to β type around
1170 K. When α type and α + β type Ti materials are held over β transus temperature in
the single β phase region, very rapid β grain growth occurs and their microstructure is
coarsened. The resultant transformed microstructure is unable to be refined by postheat
treatment. In bioceramic coatings, therefore, the deposition temperature should be low-
ered. Precursor (source gas) can be a key factor to determine the deposition temperature
in CVD. In many industrial applications, a halide precursor, particularly chloride and bro-
mide compounds, has been employed in CVD; this is termed halide CVD. Since halides are
usually thermally stable, halide CVD commonly requires a high deposition temperature,
typically more than 1300 K. On the other hand, many kinds of metal-organic compounds
(MO) have been developed these days, and MOs are more chemically reactive than halide
compounds. CVD using MO precursors (termed MOCVD) often enables deposition tem-
perature lower than halide CVD. However, the lower deposition temperature of MOCVD
often results in lower crystallinity and remaining of impurity hydrocarbons in deposited
films. This may be a drawback of MOCVD.















































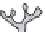
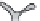

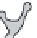



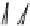














Search WWH ::

Custom Search