Biomedical Engineering Reference
In-Depth Information
atmosphere with water vapor or a partial steam pressure can be recognized as a better way
than in vacuum under the same heating conditions [59,101,120,172].
dIOC
d
2
r
=
=
k
(
1
−
IOC
)
(6.21)
t
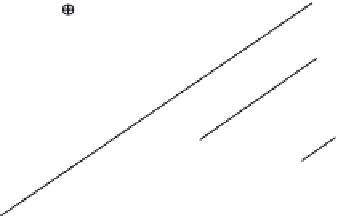
With regard to the hydrothermally crystallized conditions, Figure 6.21a shows the natu-
ral logarithmic plots for the relationship of ln(dIOC/d
t
) versus ln(1 − IOC) using the data
represented in Figure 6.15. The reaction order,
n
value, is 1.57, 1.56, and 1.53 for the hydro-
thermal crystallization at 100°C, 150°C, and 200°C, respectively. On the basis of previ-
ous reports [167,170,182] and the above-mentioned results, HA crystallization should be a
second-order reaction kinetics, which depends on the effects of heat-treatment time and
temperatures. However, the saturated steam pressures (
P
H O
2
) in the hermetical atmosphere
of the autoclaving hydrothermal treatment are 0.10 MPa (100°C), 0.48 MPa (150°C), and
1.56 MPa (200°C). Referring to the phase diagram of the CaO-P
2
O
5
-H
2
O system as shown
in Figure 6.2b [70], HA is a stable phase under an atmosphere with 500 mmHg steam pres-
], HA is a stable phase under an atmosphere with 500 mmHg steam pres-
sure, and the phase stability of HA is increased with increasing steam pressure. The effect
of
P
H O
2
70], HA is a stable phase under an atmosphere with 500 mmHg steam pres-
should also be considered as another significant factor affecting the HA crystalliza-
tion within a steam pressure environment.
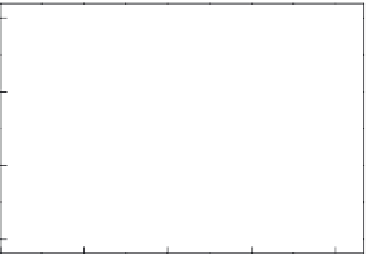
Therefore, Equation 6.22 shows the modified form that involves a saturated steam-
pressure term following the second-order reaction kinetics of Arrhenius equation. Figure
6.21b shows the 1/(1 − IOC)
1/2
versus heat-treatment time (
t
, hours) plot resulting from the
integration of Equation 6.12; here,
P
H O
2
can be thought of as a constant at each hydrother-
mal heating temperature. Since the above-mentioned saturated steam-pressure term (
P
H O
2
)
is independent of time (
t
), the slope by the least squares fitting results in Figure 6.21b is
deduced in a form of (
kP
H O
2
1 /
)/2 and the hydrothermal crystallization rate constant (
k
) for
100°C, 150°C, and 200°C hydrothermal crystallization is obtained. According to Equation
3.13, the activation energy (
E
a
) for the hydrothermal crystallization of HA can be quanti-
tatively evaluated from the Arrhenius plot of ln
k
versus 1/
T
(heating temperatures), as
shown in Figure 6.22 (where
T
is the heating temperature in Kelvin,
R
is the gas constant,
4
(a)
(b)
-2
HT100
HT150
HT200
HT100
HT150
HT200
-3
3
R
2
=
0.99
n
= 1.53
R
2
=
0.99
-4
2
R
2
=
0.99
n
= 1.56
n
= 1.57
R
2
=
0.99
R
2
=
0.99
-5
R
2
=
0.99
1
-2.5
-2.0
-1.5
-1.0
-0.5
0
3
6
9
12
ln(1 - IOC)
Heat treatment time, (
t
, (h))
FIGURE 6.21
(a) Plots of ln(dIOC/d
t
) vs. ln(1 − IOC) from natural logarithm of Equation 6.19 for hydrothermal treatment, and
(b) plots of (1 − IOC)
−1/2
vs. heat-treatment time (
t
, hours) from integration of Equation 6.22 for hydrothermal
crystallization.


























Search WWH ::

Custom Search