Biomedical Engineering Reference
In-Depth Information
1.0
0.8
0.6
0.4
HT100
HT150
HT200
0.2
0.0
0
2
4
6
8
10
12
Heat treatment time,
t
(h)
FIGURE 6.15
Index of crystallinity (IOC) for various HT-HACs.
Figure 6.16 shows typical microstructural morphologies with significant crystallization
features of the HT-HACs. It provides evidence of the differences in microscopic surface
features under various hydrothermal conditions, as illustrated in Figure 6.16a to e. It is
worth noting that new crystalline growth, indicated by arrows in Figure 6.16a, is observed
in the vicinity of the cracks and on the surface of the HT100-12h specimen. These new-
growth grains can be attributed to crystalline HA, which crystallized from the hydroxyl-
deficient structure of plasma-sprayed HACs through the replenishment of OH
−
groups.
With increasing heating temperatures and heating time of the hydrothermal treatment, the
crystalline HA experiences further grain growth with a larger crystal size and a significant
reduction in microcracks. Figure 6.16f shows the cross-sectional feature of the HT150-6h
specimen. It can be seen that the contact between lamellar boundaries are significantly
healed by the nanoscale HA crystallite after hydrothermal treatment. The microstructural
homogeneity with a reduction of these defects in the HT-HACs can be recognized as the
self-healing effect of the hydrothermal crystallization under an abundant saturated steam
environment during hydrothermal treatment.
TEM images and select-area diffraction (SAD) patterns also reveal the crystalline micro-
also reveal the crystalline micro-
structure of hydrothermally crystallized HA grains for different HA crystallinity. Figure
6.17a shows the TEM bright field image and the SAD patterns of the HT150 condition with
partial hydrothermally crystallized grains and the ACP region within coatings. The crys-
and select-area diffraction (SAD) patterns also reveal the crystalline micro-
select-area diffraction (SAD) patterns also reveal the crystalline micro-
(SAD) patterns also reveal the crystalline micro-
region within coatings. The crys-
talline grain can be recognized as HA crystals with a Ca/P ratio of about 1.61, and the ACP
region displays a Ca/P ratio of about 1.45. It can be seen that if H
2
O molecules compen-
sated for the OH
−
loss by replenishing the hydroxyl-deficient structure directly, the HACs
have a Ca/P ratio of 1.67 and become pure HA [173]. Since crystalline HA can nucleate and
grow from the ACP region [174], it is suggested that the crystallization of HA grains from
the amorphous region is responsible for the formation of these nanocrystalline grains.
Larger HA grains are observed after 200°C hydrothermal treatment (HT200), as shown in
Figure 6.17b, which represents the TEM bright field image of HA polycrystals. The TEM/
EDS analysis result represents an average Ca/P atomic ratio of about 1.66, which is close to
the theoretical value of stoichiometric HA crystal structure.
The band observation of PO
4
3−
and OH
−
detected by the Fourier transform infrared spec-
the ACP region within coatings. The crys-
ACP region within coatings. The crys-
transform infrared spec-
trometer (FT-IR) [101,175] can provide the information concerning structural features such as
the hydroxylation of HACs. In addition, the XPS analysis can further clarify the replenish-
ment of OH
−
groups and the reduction of the dehydroxylation state of hydroxyl-deficient
detected by the Fourier transform infrared spec-
the Fourier transform infrared spec-








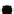






Search WWH ::

Custom Search