Biomedical Engineering Reference
In-Depth Information
of HA or formation of mineralized matrix by osteoblasts (Wu et al. 2002). The most fre-
quently used sols are obtained by hydrolyzing acetic acid and acetilacetone complexed
titanium alkoxides. Shimizu et al. have successfully fabricated anatase titania thin films
on glass and various organic substrates at 40
o
C to 70
o
C using aqueous solutions of tita-
nium tetrafluoride (TiF
4
) (Shimizu et al. 1999). Wu et al. have also fabricated anatanse tita-
nia coatings on titanium. In vitro experiments disclose that the coatings have good bone
bonding ability (Wu et al. 2002).
Controlling the grains size of the titania particles can be accomplished by two tech-
niques. The most frequently used method to suppress the agglomeration and growth of
nanoparticles is the substitution of the surface hydroxyl groups by other functional groups
such as organic complex molecules that do not condense like OH
−
and that can eventually
provide the redispersibility of the powdered xerogel (Kaminski et al. 2005). Nevertheless,
subsequent heat treatments that should be carried out in order to remove the organic
groups may strongly affect the regular nanostructure of the prepared film.
Shen et al. (2005) have reported the fabrication of hydrophobic nanoporous titania by the
sol-gel and dip method. However, it is generally accepted that a hydrophilic surface ben-
efits cell attachment but the biological performance of a hydrophobic surface is unknown.
It is noted that this hydrophobic surface can greatly improve the corrosion resistance of
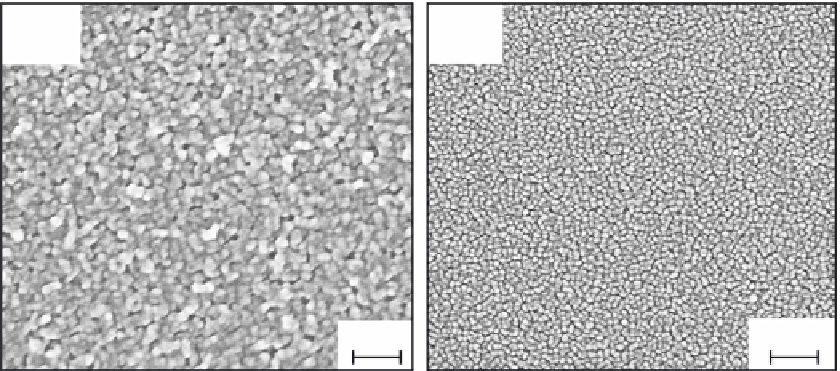
the coated metal as demonstrated by Shen et al. (2005). As the titania coatings produced by
the sol-gel method are usually prone to cracking during densification and crystallization,
immersion in boiling water for 5 to 10 min can alleviate the problem, and the morpholo-
gies of the coating before and after exposure in water are exhibited in Figure 5.24. Kuyyadi
has fabricated nanocrystalline titania coatings using titanium isopropoxide {Ti(OC
3
H
7
)
4
}
and ethanol. Water is added for hydrolysis and polycondensation and nitric acid is used
to control precipitation (Biju and Jain 2008). The coating is then annealed and Figure 5.25
shows the typical morphology of the sol-gel coating after heating at 550°C. The anatase
titania coating with a grain size of about 25 nm is observed from the picture and the grain
size increases with sintering temperature.
(a)
(b)
100 nm
100 nm
FIGURE 5.24
SEM images of nano-TiO
2
coated 316L stainless steel by sol-gel processing (a) before hydrothermal posttreat-
ment and (b) after hydrothermal treatment. (From Shen et al.,
Electrochim. Acta
, 50, 5083-5089, 2005. With
permission.)
Search WWH ::

Custom Search