Biomedical Engineering Reference
In-Depth Information
metal to the oxide/electrolyte interface and dissolve in the electrolyte. The growth rate of
oxide at the metal and oxide interface and the dissolution rate of oxide at the pore-bottom/
electrolyte interface are ultimately equal. Therefore, the thickness of the barrier remains
unchanged, although it migrates further into the metal leading to increasing tube length.
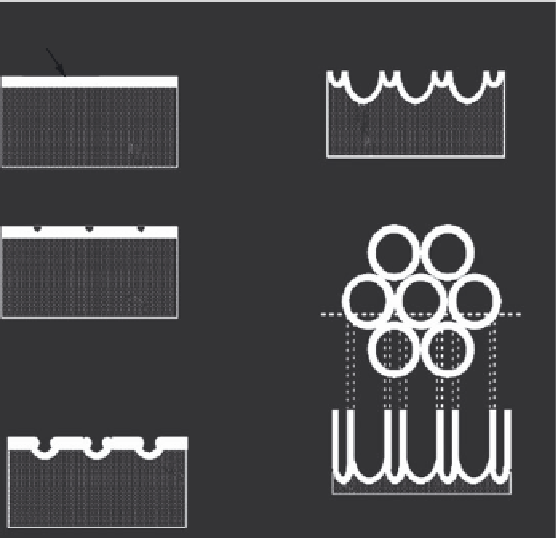
The SEM results demonstrate that formation of small pits at the interpore region eventu-
ally leads to pore separation and tube formation. The thickness of the nanotubes ceases to
increase when chemical dissolution of the oxide at the mouth of the tube equals to the rate
of inward movement of the metal/oxide interface. This process is schematically illustrated
in Figure 5.6 (Grimes and Mor 2009).
Chemical dissolution plays a key role in the formation of self-organized nanotube arrays.
It reduces the thickness of the oxide layer (barrier layer) and keeps the electrochemical
etching active. No nanotubes can be fabricated when chemical dissolution is too high or
too low. The electrochemical dissolution rates depend on the applied potential as well as
electrolyte concentrations. When electrochemical etching proceeds faster than chemical
dissolution, the thickness of the barrier layer increases leading to reduced electrochemical
etching to the rate determined by the chemical dissolution. In the F
−
containing system,
the chemical dissolution rate is controlled by the F
−
concentration and pH of the electrolyte.
Chemical dissolution increases with increased F
−
and H
+
concentration. The anodization
potential at which nanotubes form is related to the F
−
concentration, with higher potentials
required for an electrolyte with higher F
−
concentration (Grimes and Mor 2009).
(d)
(a)
Pores
Voids
Oxide
Barrier layer
Metal
(b)
(e)
Metal
Tube
(c)
Pores
Void
Barrier layer
Barrier layer
FIGURE 5.6
Schematic illustration of nanotube formation process. (a) Oxide layer formation, (b) pit formation on oxide layer,
(c) growth of pit into scallop shaped pores, (d) metallic region between pores undergoes oxidation and field
assisted dissolution, and (e) fully developed nanotubes with a corresponding top view. (From Grimes, C.A.,
Mor, C.K.,
TiO
2
Nanotube Arrays. Synthesis, Properties, and Applications
, Springer Science, New York, NY, 2009.
With permission.)









Search WWH ::

Custom Search