Biomedical Engineering Reference
In-Depth Information
of the powders, have been systematically investigated (Tong et al. 1996; Cheang et al. 1996b;
Liu et al. 1994).
The most widely used and well-established method for HA powder synthesis is wet
chemical route, which employs the reaction of calcium hydroxide with orthophosphoric
acid according to the reaction:
10Ca(OH)
2
+ 6H
3
PO
4
→ Ca
10
(PO4)
6
(OH)
2
+ 18H
2
O
(4.1)
Detailed description of the procedure for the production of the powder was reported (Kweh
2000). Parameters such as temperature, pH, rate of mixing reactants, and maturation time
have been carefully controlled to ensure production of an apatite as close as possible to the
stoichiometric HA. In addition, special care must be taken to ensure a slow drip rate of the
acid onto the alkaline solution to favor the formation of PO
4
3−
ion and the introduction of
OH
-
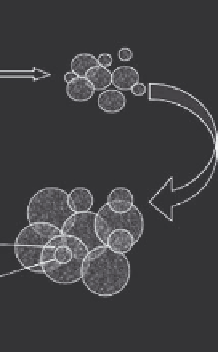
in the lattice. An overview of the synthesis procedure is given schematically in Figure
4.4. It consists primarily of three steps: (1) preparation of the solution, (2) precursor synthe-
sis, and (3) high temperature treatment to consolidate powders.
In order to prepare specific powders, two steps have to be mastered: the slurry stability
and the spray drying operating conditions. During spray drying, the solution is extracted
from a feed tank and passed through the spray atomizer. It is a result of many interwoven
complex mechanisms. The feed materials are either water-based suspensions with air as
drying gas or organic solvent-based suspensions (usually ethanol) with nitrogen as drying
gas. Compressed air with a selected pressure is used during spraying. The atomized liquid
is rapidly dried by a coaxial flow of air that has been previously preheated to a specific
temperature. The dried powder is cyclone separated from the flowing airstream.
An important study that has to be carried out especially when nonstoichiometric apatite
powder is synthesized is its thermal stability. Only pure HA powder remains stable up to
its melting temperature but nonstoichiometric apatite would undergo irreversible decom-
position reactions through a wide range of temperatures that can go up to 1000°C. Above
this temperature, the reversible decomposition of stoichiometric HA takes place. Rey and
Precursor powder:
A and B are well
mixed in one particle
Liquid:
A and B
Spray dry
the liquid
High temperature
treatment
Nanostructured powder
FIGURE 4.4
Schematic illustration of the wet chemical reaction approach. (Li, H., unpublished data, 2004.)

Search WWH ::

Custom Search