Geology Reference
In-Depth Information
9.5.2.2 Concentration exergy
Once a mineral has been created it mixes with others to form rocks, which in turn are
combined with other rocks so as to form a deposit (stage IV of the mineral formation
described in Sec. 9.2). The minimum theoretical work needed to concentrate a
substance from an ideal mixture of two components is given by the concentration
exergy (b
c
), as in Eq. (9.30), which derives from the expression of the entropy of
mixing (Sec. 9.5).
b
ci
= RT
0
(1 x
i
)
x
i
lnx
i
+
ln(1 x
i
)
(9.30)
The difference obtained in the concentration exergies of a mineral concentration
in a mine (x
m
)
7
and that of the average concentration in the Earth's crust, (x
c
)
8
is
effectively the minimum amount of energy that Nature had to spend to bring the
minerals from the concentration present in the dispersed state of Thanatia to that
found in a mine. The exergy of a mineral increases along with its concentration.
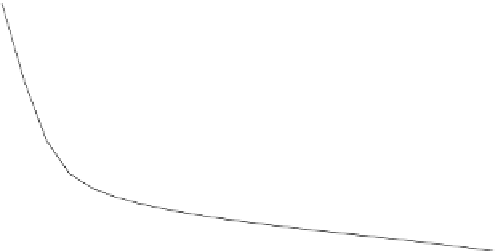
Such growth is not linear, since according to the Second Law, reflected in Eq. (9.30)
and represented in Fig. 9.5, the effort required to excavate a mineral from a mine
follows a negative logarithmic pattern with its ore grade. So, as the ore grade tends
to zero, the energy needed to extract the mineral tends towards infinity. It is thus
this component of the mineral exergy which makes it a more realistic measure of
magnitude than mass, as pointed out by Wall (1977). Furthermore, this fact invali-
dates the statement of Brooks and Andrews (1974) that the exhaustion of minerals
is ridiculous because the entire planet is composed of minerals.
35
b
c
, MJ/kmol
30
25
20
15
10
5
0
0.00001
0.15
0.4
0.65
0.9
x
i
Fig. 9.5 Exergy required for separating a substance from a mixture, according to Eq. (9.30).
7
x
m
replaces x in Eq. (9.30) for obtaining the concentration exergy of the mineral in the mine.
8
x
c
replaces x in Eq. (9.30) for obtaining the concentration exergy of the mineral provided in the
baseline.












Search WWH ::

Custom Search