Geology Reference
In-Depth Information
ratio
P0
2
ratio
O
1
-
G
0
(kJ/mol O
2
)
1
M
100
M
M
1
200
M
M
300
M
400
M
B
H
500
M
CO
M
C
M
B
M
600
700 -
800
B
900
B
M
1000
M
M
1100
1200 -
0
º
C
Absolute
0 Kelvin
200
400
600
800
1000
1200
1400
1600
1800
2000
2200
2400
ratio
ratio
Ag
2
O: 4Ag + O
2
2Ag
2
O
Fe
3
O
4
: 4Fe
3
O
4
+ O
2
6Fe
2
O
3
CO
2
: C + O
2
CO
2
CO: 2C+ O
2
2CO
Cu
2
O: 4Cu + O
2
2Cu
2
O
FeO: 2Fe + O
2
2FeO
ZnO: 2Zn + O
2
2ZnO
Cr
2
O
6
: 4/3Cr + O
2
2/3Cr
2
O
6
MnO: 2Mn + O
2
2MnO
SiO
2
: 2Si + O
2
2SiO
2
TiO
2
: 2Ti + O
2
2TiO
2
Al
2
O
3
: 4/3Al + O
2
2/3 Al
2
O
3
MgO: 2Mg + O
2
2MgO
CaO: 2Ca + O
2
2CaO
Line code
Element
Oxide
Condensed
Condensed
Gaseous
Gaseous
Condensed
Gaseous
Melting Point of element M
Melting Point of oxide M
Boiling Point of element B
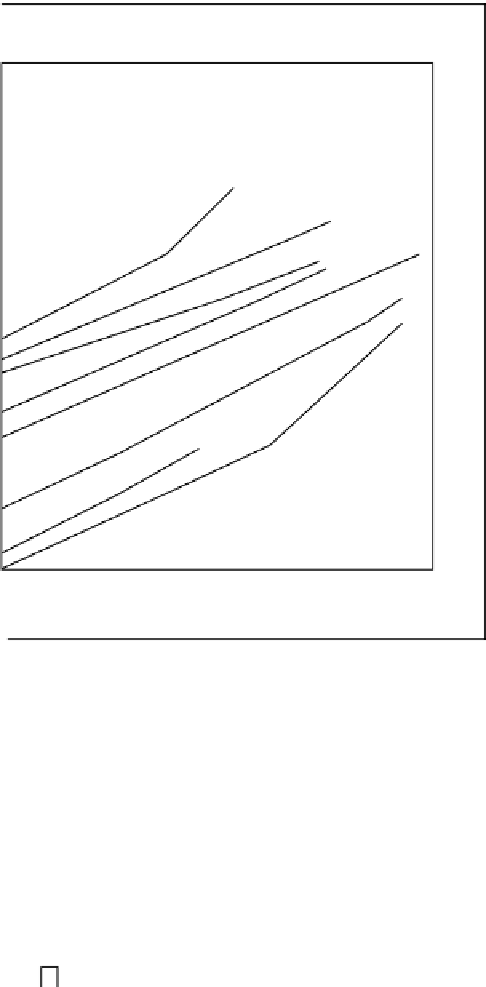
Fig. 9.3 Standard Gibbs free energy of oxide formation as a function of temperature. Redrawn
from Richardson and Jeffes (1948) and Draken and Gurry (1953)






































































































Search WWH ::

Custom Search