Geology Reference
In-Depth Information
Open Pit Mining
(6% Bastnasite)
Crushing and Grinding
(90% to < 74µm)
Conditioning Tank
(Chemicals and Steam)
Depressed
gangue
calcite, fluorite
and barite
Depressed silicates,
iron and niobium
oxides at the bottom
to be recovered
Selective
Froth
Floatation
Thickening
and
Desliming
Bulk Floatation
Bastnasite
(60-70% REO)
Thickening, Filtering
and Drying
HCl (10%)
Leaching
RECl
3
85-90%
Thickening
Filtering
Calcining
Ca and Sr Carbonates
CO
2
Removal
CeO
2
concentrate
Fig.8.12FlowdiagramforBastnaesite'sphysico-chemicalbeneficiation
8.13.6 Isolation ofREElements
Separation and purification equipment falls under fine chemistry. Flexible and so-
phisticated apparatuses are used for the treatment of varied REO composition feeds
and products. These processes require large amounts of power and reagent inputs
and contribute to some '70% of the overall operating costs (Gibson and Parkinson,
2011). Therefore, it is the separation and purification stage which provides the
major added value to the final price of an isolated REO and/or REE.
The isolation of REE from each other requires further processing which takes
advantage of their small chemical and physical differences. Fractional crystallisa-
tion, fractional precipitation, solvent extraction and ion exchange are all empirically
used techniques. The latter two are the most common. Each one has its own ad-
vantages and disadvantages pertaining to the composition of the final product, the
degree of separation from other REE, the economic viability or process e
ciency.
Slight variations in basicity as well as selective oxidation or reduction provide the
fundamentals of separation for all of them. Therefore, the individual separation of
REE begins with the oxides, given that they easily hydrolyse in water and simply
precipitate. As it happens with the alkaline and alkaline-earths oxides, they are dif-
ficult to reduce because they form stable oxides of the trivalent ion, M
+3
. Cerium,
as an exception, can be oxidised into the stable salts of Ce
+4
whilst Pr and Tb
permit the M
4+
state in its more instable form. Sm, Eu and Y b can also accept a
reduction to divalent ions and become strong reducing agents.
As explained, cerium is the most abundant REE and is first removed by dry-
ing hydroxides with hot air or any other form of selective oxidation that converts
Ce(III) into the less basic Ce(IV ). The treatment of hot dilute acid selectively pre-
cipitates the cerium oxide. Eu(III) may be selectively reduced with a zinc amalgam
in a chloride solution and then precipitated as a sulphate. Europium can also be
electrolytically reduced. Additionally, Eu can be separated along with Sm and Y b in












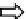



















Search WWH ::

Custom Search