Biomedical Engineering Reference
In-Depth Information
1
0.9
0.8
0.7
0.6
0.5
0.4
0.3
0.2
= 0
= 0.1
= 1
0.1
0
1
1
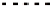
FIGURE 17.7
Effect of effectiveness factor for spherical geometry.
Instead of having one differential equation (for isothermal cases), we now have two differ-
ential
Eqns (17.22) and (17.65)
. If the heat of reaction is constant, i.e. not a function of temper-
ature (and pressure, or concentration),
Eqn (17.65)
(
DH
R,A
)
Eqn (17.22)
yield,
d
d
x
k
eT
S
d
T
d
x
ðDH
R
;
A
Þ
d
d
x
D
eA
S
d
C
A
d
x
¼ 0
(17.66)
Integrate
(17.66)
once, we obtain
k
eT
S
d
T
d
x
ðDH
R
;
A
ÞD
eA
S
d
C
A
d
T
d
x
ðDH
R
;
A
ÞD
eA
S
d
C
A
d
x
k
eT
S
d
x
¼
(17.67)
x¼0
When
x
0 (i.e. the deepest location inside the porous catalyst the reactant diffuses
to), both heat flux and mass transfer flux are zero. Therefore, the right-hand side of
Eqn
(17.67)
is zero. Dividing through by
S
and integrating the resultant equation, we obtain
¼
0or
C
A
¼
k
eT
T ðDH
R
;
A
ÞD
eA
C
A
¼ k
eT
T
S
ðDH
R
;
A
ÞD
eA
C
AS
(17.68)
where
T
S
is the temperature on the external surface of the catalyst. We have assumed constant
k
eT
and
D
eA
to arrive at
Eqn (17.68)
. Rearranging
Eqn (17.68)
, we obtain
T ¼ T
S
þ
ð
DH
R
;
A
Þ
D
eA
k
eT
ðC
AS
C
A
Þ
(17.69)
Therefore, the temperature is linearly related to the concentration and thus the internal effec-
tiveness factor can be evaluated by solving one differential
Eqn (17.22)
with temperature
given by
Eqn (17.69)
. This significantly reduced the computing power needed.




































































































































































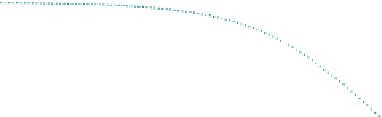











































Search WWH ::

Custom Search