Biomedical Engineering Reference
In-Depth Information
Therefore, the mass rate supply of A to a CSTR is linearly related to the conversion and/or
concentration,
Eqn (16.6)
or
Eqn (16.5)
.
Let us now look at the right-hand side in the context of molar (or mass) balance. It is the
molar (mass) consumption rate (or negative molar generation rate) of A. That is
MC
A
¼r
A
(16.7)
The mass balance equation can thus be expressed as the mass supply rate of A to the reactor
(MS
A
) equals to the mass consumption rate of A (MC
A
) in the reactor. The solution of a CSTR
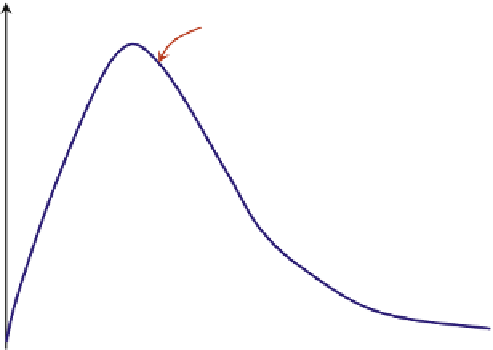
problem is thus visually illustrated in
Fig. 16.3
: on the plane of “mass change rate of A” versus
“concentration of A” (or “fractional conversion of A”), the steady-state solution is the intercept
between the “mass supply rate line” and the “mass consumption rate curve”. That is
MC
A
¼
MS
A
(16.8)
The steady-state solutions are characterized by the intercepts of the mass consumption
curve and the mass supply line. In the schematic diagram shown in
Fig. 16.3
, there are three
steady-state solutions. The number of steady-state solutions in this case is determined by the
shape of mass consumption curve as one can infer from
Fig. 16.3
. MSS solutions exist if there
are values of the independent variable (C
A
>
0or0
<
f
A
<
1) exists such that
Eqn (16.8)
and
dMC
A
d
C
A
¼
dMS
A
d
C
A
(16.9a)
or
dMC
A
d
f
A
¼
dMS
A
d
f
A
(16.9b)
hold true for some values of C
A
or f
A
by varying feed concentration C
A0
or dilution rate D as
illustrated in
Figs. 16.4 and 16.5
. The locations (or values of
C
A0
¼ C
A0
or D
¼
D*) at which
MC
A
or
-r
A
DC
A0
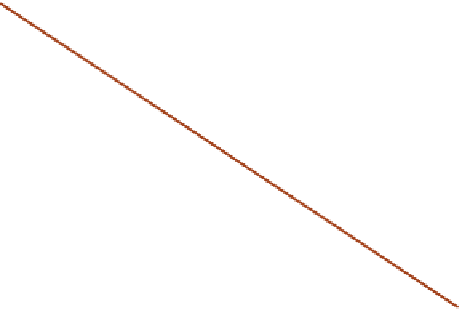
I
II
MS
A
Slope =
-D
III
0
C
Ae
C
A0
0
C
A
FIGURE 16.3
Schematicmass balances in a chemostat or CSTR. Themass consumption rate ofA,MC
A
, is identical to
the rate of reactionof A,
r
A
, in the CSTR operating conditions, whereas themass supply rate of A (feed rate of Asubtract
the rate of A letting out of the CSTR), MS
A
, depends linearly on the concentration (for a constant density reactor).












Search WWH ::

Custom Search