Biomedical Engineering Reference
In-Depth Information
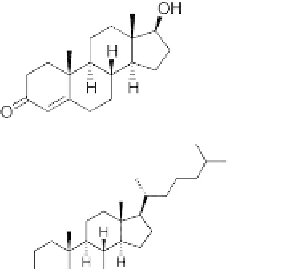
Testosterone (a male sex hormone)
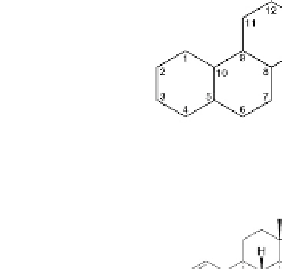
Location of carbon in ring structure
Cholesterol (precursor for many steroids including
testosterone and cortisone)
Estrogen (a female sex hormone)
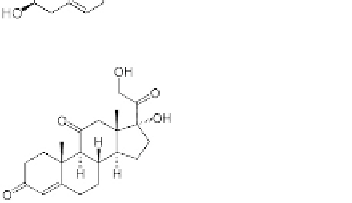
Cortisone (an adrenocortical hormone)
Progesterone
FIGURE 2.29
Examples of important steroids. The basic numbering of the carbon atoms in these molecules is
also shown.
2.3.6. Nucleic Acids, RNA, and DNA
Nucleic acids play a central role in the reproduction of living cells. DNA stores and
preserves genetic information. RNA plays a central role in protein synthesis. Both DNA
and RNA are large polymers made of their corresponding nucleotides.
Nucleotides are the building blocks of DNA and RNA and also serve as molecules to store
energy and reducing power. The three major components in all nucleotides are phosphoric
acid, pentose (ribose or deoxyribose), and a base (purine or pyrimidine).
Figure 2.30
depicts
the structure of nucleotides and purine
e
pyrimidine bases. Two major purines present in
nucleotides are adenine (A) and guanine (G), and three major pyrimidines are thymine (T),
cytosine (C), and uracil (U). DNA contains A, T, G, and C, and RNA contains A, U, G, and
C as bases. It is the base sequence in DNA that carries genetic information for protein synthesis.
This information is expressed in its owner and passed on from one generation to another.
The triphosphates of adenosine (and, to a lesser extent, guanosine) are the primary energy
currency of the cell. The phosphate bonds in adenosine triphosphate (ATP) and guanosine
triphosphate (GTP) are high-energy bonds. The formation of these phosphate bonds and their
hydrolysis is the primary means by which cellular energy is stored and used. For example,
the synthesis of a compound that is thermodynamically unfavorable can be accomplished
in conjunction with ATP hydrolysis to ADP (adenosine diphosphate) or to AMP (adenosine
monophosphate).
The coupled reactions can proceed to a much greater extent, since the free-energy change
becomes much more negative. In reactions that release energy (for example, oxidation of



Search WWH ::

Custom Search