Biomedical Engineering Reference
In-Depth Information
TABLE 2.6
Examples of Common Fatty AcidsdCont'd
Acid
Structure
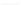
PGE
2
O
OH
O
HO
OH
The value of n is typically between 12 and 20. Unsaturated fatty acids contain
double
e
C
]
C
e
bonds, such as oleic acid.
Oleic acid : CH
3
ð
CH
2
Þ
7
CH
¼
CH
ð
CH
2
Þ
7
COOH
Sometimes, the location of the double bond nearest to the end opposite to the carboxyl
group is of interest. For example, oleic acid's double bound is at the 9th carbon (opposite
to COOH) and it is an
-9 fatty acid.
Fats are esters of fatty acids with glycerol. The formation of a fat molecule can be repre-
sented by the following reaction:
u
O
O
H
2
COH
HO
C
(CH
2
)
n
1
CH
3
H
2
CO
C
(CH
2
)
n
1
CH
3
O
O
+
HCOH
+
HO
C
(CH
2
)
n
2
CH
3
3 H
2
O
HCO
C
(CH
2
)
n
2
CH
3
O
O
H
2
COH
HO
C
(CH
2
)
n
3
CH
3
H
2
CO
C
(CH
2
)
n
3
CH
3
(Glycerol)
(Fattyacids)
(Triglyceride)
Phosphoglycerides have similar structures to fats, the only difference being that phosphoric
acid replaces a fatty acid and is esterified at one end to glycerol.
Membranes with selective permeability are key to life. Cells must control the entry and
exit of molecules. Phospholipids are key components, but membranes contain large amounts
of proteins. Biological membranes are based on a lipid bilayer. The hydrophobic tails of the
phospholipids associate with each other in the core of the membrane. The hydrophilic
heads form the outsides of the membrane and associate with the aqueous cytosol or the
aqueous extracellular fluid. Some proteins span across the membrane, while others are
attached to one of the surfaces. Membranes are dynamic structures, and lipids and proteins
can diffuse rapidly. Typical membrane phospholipids include phosphatidylcholine, phos-
phatidylserine, phosphatidyl glycerol, and phosphatidyl inositol. The phosphatidyl group











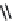





































Search WWH ::

Custom Search