Biomedical Engineering Reference
In-Depth Information
C=O
C=O
C
C=O
C=O
C
C
C
C
H
C
OH
HO
C
H
H
OH
H
2
COH
CH
2
OH
CH
2
OH
Counter
clockwise
H
OH
L-
enantiomer
D-
enantiomer
FIGURE 2.17
Representation of enantiomers.
(one chiral center, or one asymmetric carbon that has at least three different units or groups
bonded to it). The light rotation produced by a sugar with multiple chiral centers will be
determined by the combined effects of all the chiral centers. The
D
-enantiomers is best
described by the right-hand gesture as illustrated by the two figures on the left in
Fig. 2.17
, where the thumb points toward the CHO end group, and the natural
bending of the other four fingers indicating the direction in which the end groups
(OH to CH
2
OH to H) are ordered as counter-clockwise (opposite to polarized light). For
L
-enantiomers, left-hand rule applies (end groups are arranged OH, CH
2
OH, and H clock-
wise). The sugars listed below consist of mostly of those sugars naturally present. The
L
form generally plays a minor role in biological systems, with the exceptions of
L
-arabinose,
L
-rhamnose, and
L
-fucose.
2.3.2.1.1. ALDOSES
An aldose is a sugar having a reducing end group: CHO. The C
]
O double bond is located
on the last (or first) carbon when the sugar molecule is represented linearly.
a.
D
-hexoses
These sugars (hexoses) all have the same chemical formula (C
6
H
12
O
6
) and even the same
number, type, and sequence of bonds. They only differ by the spatial arrangement about
onesinglecarbonatominthechain.Theyareepimers.Intheabove,theonlydifference
is one of the orientations of the OH (hydroxyl) groups (left or right). Although most
epimers' physical and chemical properties are the same, their biological activities can be
quite different.
CHO
HOCH
CHO
HCOH
CHO
HOCH
CHO
HOCH
CHO
HCOH
CHO
HOCH
CHO
HCOH
CHO
HCOH
HOCH
HCOH
HCOH
HOCH
HOCH
HCOH
HCOH
HOCH
HCOH
HOCH
HOCH
HCOH
HCOH
HCOH
HCOH
HCOH
HCOH
HCOH
HOCH
HCOH
HCOH
HCOH
HOCH
HCOH
CH
2
OH
CH
2
OH
CH
2
OH
CH
2
OH
CH
2
OH
CH
2
OH
CH
2
OH
CH
2
OH
D
-allose
D
-altrose
D
-glucose
D
-gulose
D
-mannose
D
-galactose
D
-idose
D
-talose










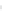

















































Search WWH ::

Custom Search