Biomedical Engineering Reference
In-Depth Information
If the adsorption is truly physisorption, the adsorbed adsorbate may be considered to be
equivalent to “liquefied” or “solidified” adsorbate, in which case,
p
A
p
A
ap
A
¼ b ¼
(9.74)
One parameter can be eliminated, and the adsorption isotherm is reduced to
1
þ N
1
p
A
p
A
N
p
A
p
A
1
n
As;N
¼ n
s1
c
p
A
(9.75)
1
1
p
A
c
p
A
Nþ1
p
A
p
A
p
A
p
A
ð
1
cÞ
p
A
which is applicable only to physisorptions.
Assuming that there is an infinite number of layers of adsorbate possible (
N
),
Eqn
/ N
(9.75)
is reduced
n
s
c
p
A
p
A
1
1
n
As;
N
¼
(9.77)
p
A
p
A
p
A
p
A
ð
1
cÞ
Eqns
(9.74), (9.75), and (9.77)
are not applicable to chemisorptions because the interactions
between adsorbate and adsorbent are stronger. Adsorbate molecules can be packed together
on the adsorbent surface much tighter than the solid state of the adsorbate, which result in
a much smaller
p
A
than actual vapor pressure if these equations were used.
In summary, the adsorption isotherms exhibit different dependence on the bulk adsorbate
concentration:
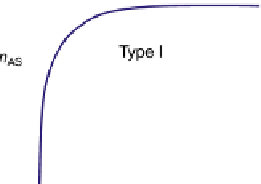
Type I isotherm can be either chemisorptions or physisorption. There is a saturation
adsorption on the adsorbent surface. The capacity of adsorption is limited by the available
active centers on the adsorbent surface. It is the simplest kind and widely seen in solid
catalysts.
Types II and III, on the other hand, are only seen for physisorptions, where a saturation
bulk phase adsorbate concentration exists. There is no limit on the capacity of the adsorption


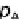




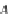
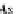
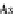

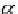

















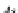
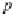

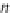
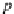

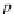

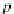
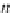

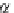
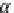

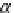
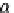

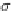




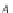
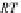
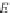

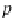

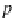
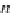

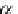
























Search WWH ::

Custom Search