Biomedical Engineering Reference
In-Depth Information
Type II
Type III
Type I
n
AS
n
AS
n
AS
0
0
0
p
°
p
°
p
A
p
A
p
°
p
A
Type V
Type IV
n
AS
n
AS
0
0
p
A
p°
p
A
p°
FIGURE 9.12
Classification of adsorption isotherms based on the adsorption of gas on a solid material. The
horizontal axes are the partial pressure of the adsorbate gas, and the vertical axes are the amount of gas adsorbed on
the surfaces.
At this point, we know that the adsorption can be described using concentrations as we
have done so far and equally well with partial pressures as discussed earlier. Whether the
adsorption is chemisorptions or physisorption, the adsorption and desorption rates are
similar: adsorption requires the adsorbate molecules to get close enough to the adsorption
site or active centers and the desorption occurs when the adsorbed molecules leave the adsor-
bent surface.
9.1.4.2. Multilayer Adsorption of Single Species
Fig. 9.13
shows a schematic of multilayer adsorption, which is a direct extension of
Fig. 9.7
.
The similarity of the model leads us to similar analysis. As we have discussed for bi-layer
adsorption, in each layer, there is a dynamic
equilibrium with the bulk gas phase. The
adsorption is governed in a manner similar
to
Eqn (9.1a)
. The adsorbate molecule can
only be adsorbed on to a site on a layer if
the site on the lower layer is occupied.
On
layer
i
4
3
the
top
layer,
the
adsorp-
2
1
tion
e
desorption is governed by
0
¼ r
ad;N
¼ k
N
ðq
N1
q
N
Þp
A
k
N
q
N
(9.56)
FIGURE 9.13
Multilayer surface adsorption model
for the BET isotherm.






































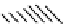
































Search WWH ::

Custom Search