Biomedical Engineering Reference
In-Depth Information
200
150
100
50
Langmuir Isotherm
Temkin Isotherm
0
0
20
40
60
80
100
C
A
, g/m
3
FIGURE E9-2.2
Temkin isotherm model fit as compared with the Langmuir isotherm fit for the adsorption of
phenol on activated carbon.
9.1.4. Adsorption at High Surface Coverage
Thus, so far we have examined only the process of ideal (and nonideal) chemisorptions
where the adsorbate molecules only interact with the adsorbent surfaces and no interactions
with adjacent adsorbate molecules. At high surface coverage, this idealization is no longer
valid. It is well known that adsorbate molecules “pack” very closely on the adsorbent surface,
which result in significant adsorbate
e
adsorbate interactions. We next continue our discus-
sion on the nonideal chemisorption to high surface coverages where adsorbate
e
adsorbate
molecule interactions must be accounted for.
To further our consideration, let us once again recall the collision theory of gas molecules
(atoms) with surfaces:
s
RT
2pM
A
Z
cT
ð
A
;
surface
Þ¼N
AV
C
A
(6.21)
which is equivalent to
N
AV
p
A
Z
cT
ð
A
;
surface
Þ¼
p
2pM
A
RT
(9.45)
Therefore, adsorption (and reaction in general) can be considered either in concentrations or
in partial pressures for gas adsorption. Now let us make up a little picture of the surface
(which is two-dimensional) and adsorbed molecules that the collisions are involved with,
as in
Fig. 9.8
.











































































































































































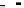















Search WWH ::

Custom Search