Biomedical Engineering Reference
In-Depth Information
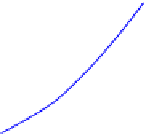
the other hand, very difficult experimentally, as higher temperature usually results in lowers
adsorption isotherm equilibrium constant
K
A
. The decrease in
K
A
requires much higher
concentration of
C
A
in order to achieve the same level of adsorption as depicted by
Fig. 9.5
, where concentration is plotted on log scale.
9.1.2. Idealization of Nonideal Surfaces
It is not hard to imagine that no real surface could have the potential-energy distribution
as depicted in
Fig. 9.2
nor is it reasonable to expect that adsorbate molecules on the surface
would not interact with each other. Somewhat more plausible is the limitation of monolayer
adsorption. In any of these events, one would expect to observe some distribution of energies
of interaction with the surface which might be correlated with surface coverage. Since the
strongest interactions would occur on the nearly unoccupied surface, the experimental obser-
vation would be that of decreasing heat of chemisorption with increasing coverage. It is not
possible to distinguish from this information alone whether energetic inhomogeneity of the
surface or adsorbate interactions are the cause, but in most of the practical applications with
which we are concerned, this amount of detail is not necessary. In the following discussion,
the case of an inhomogeneous surface shall be considered, using the specific example of non-
dissociative adsorption of a single species for illustration.
Consider that the surface may be divided into a number of groups of sites, each character-
ized with a similar heat of chemisorption and so capable of being represented by a Langmuir
isotherm. The fractional coverage of each of these for a single adsorbate A is
K
Ai
C
A
q
Ai
¼
(9.18)
1
þ K
Ai
C
A
n
max
n
AS
T
1
T
2
T
3
T
4
0.01
0.1
1
10
100
C
A
FIGURE 9.5
Variation of adsorption isotherm with temperature following Langmuir adsorption.





































































































































































































































































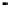




















Search WWH ::

Custom Search