Biomedical Engineering Reference
In-Depth Information
0.20
0.18
0.16
0.14
0.12
0.10
0.08
0.06
0.04
0.02
0.00
0
10
20
30
40
50
60
S
, mmol/L
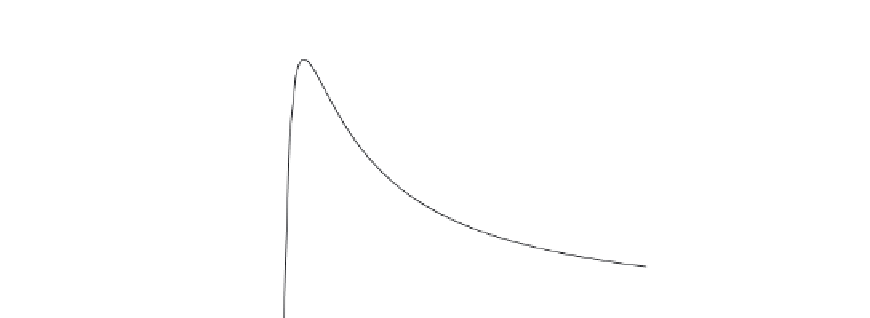
FIGURE E8-4.1
(a) Show that the rate law for substrate inhibition is consistent with the data shown in the
Fig. E8-4.1
for r
P
versus the substrate concentration of S.
(b) If this reaction is carried out in a fluidized CSTR that has a working volume of 5000 L, to
which the volumetric flow rate is 15 L/min and the feed concentration of the substrate is
60 mmol/L, determine the possible conversion of substrate to the desired product. Since
the enzyme is immobilized on the solid particle, assume that there is no loss of the
enzyme to the effluent.
(c) What would be the effluent substrate concentration if the enzyme loading is 25% lower?
Solution. For substrate inhibition, the mechanism is given by
k
1
E
þ
S
E
$
S
(E8-4.1)
%
k
1
k
2
P
E
$
S
þ
E
(E8-4.2)
/
k
3
k
3
E
$
S
þ
S
S
$
E
$
S
(E8-4.3)
%
The inhibition step, reaction (E8-4.3), renders the enzyme ineffective and thus slows down
the overall reaction rate of utilizing the substrate. The rate of formation for P is given by
(E8-4.4)
where the intermediate concentration can be obtained through pseudosteady-state hypoth-
eses for the intermediates:
r
P
¼ k
2
½
E
$
S
0 ¼ r
E
$
S
¼ k
1
½
E
½
S
k
1
½
E
$
S
k
2
½
E
$
S
k
3
½
E
$
S
þk
3
½
S
$
E
$
S
(E8-4.5)
0 ¼ r
S
$
E
$
S
¼ k
3
½
E
$
S
½
S
k
3
½
S
$
E
$
S
(E8-4.6)



































































































































Search WWH ::

Custom Search