Biomedical Engineering Reference
In-Depth Information
100
90
80
60 hour
s
1 hour
70
1
0 hours
60
50
40
30
20
10
0
10
20
30
40
50
60
T
, °C
FIGURE 8.16
Effect of temperature on the activity of an enzyme. Here we have assumed a value of E
a
¼
46 kJ/
mol, E
d
¼
10
49
/h. The increase in maximum rate is due to the increase in the activity via
Arrhenius law, while the descending of the curve is due to the dominance of the thermal denaturation. The enzyme
activity or relative maximum rate is averaged for a total exposure of 1-hour, 10-hour, and 60-hour to the temper-
ature, which is shown in Eqn
(8.92)
.
301 kJ/mol, and k
d0
¼
1.0
where E
d
is the deactivation energy (kJ/mol). Consequently,
e
k
d
t
E
a
RT
r
max
¼ k
20
½
E
0
exp
(8.91)
The activity or average maximum rate for a total exposure time of t is thus given by
R
t
r
max
d
t
t
¼ k
20
½
1
e
k
d
t
k
d
t
E
a
RT
0
r
max
¼
E
0
exp
(8.92)
The activation energies of enzyme-catalyzed reactions are within the 15
e
85 kJ/mol range
(mostly about 46 kJ/mol). Deactivation energies E
d
vary between 170 and 550 kJ/mol
(mostly about 300 kJ/mol). That is, enzyme denaturation by temperature is much faster
than enzyme activation. A rise in temperature from 30
e
40
C results in a 1.8-fold increase
in enzyme activity but a 45-fold increase in enzyme denaturation. Variations in temperature
may affect both r
max
and K
m
values of enzymes.
Figure 8.15
is plotted using Eqn
(8.92)
.
8.2.6. Insoluble Substrates
Enzymes are often used to attack large, insoluble substrates such as woodchips (in
biopulping for paper manufacture) or cellulosic residues from agriculture (e.g. cornstalks).
In these cases, access to the reaction site on these biopolymers by enzymes is often limited
by enzyme diffusion. The number of potential reactive sites exceeds the number of enzyme
molecules. This situation is opposite to that of the typical situation with soluble substrates,

























































































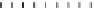







































































































































































































Search WWH ::

Custom Search