Biomedical Engineering Reference
In-Depth Information
No inhibition
r
P
Competitive inhibition
1
2
r
max
[S] =
K
m
K
m
(
1 +
)
[I]
K
I
[S] =
0
0
[S]
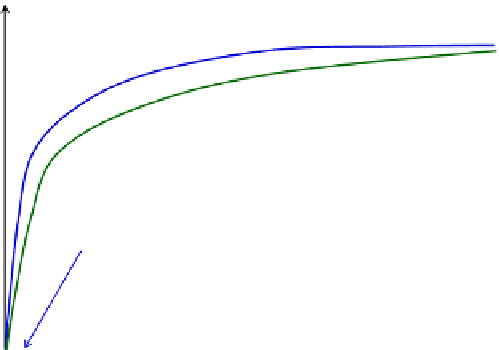
FIGURE 8.11
Comparison of Michaelis
e
Menten and competitive inhibition enzyme kinetics.
Figure 8.11
shows a comparison between the rate with the competitive enzyme inhibition and
that of the Michaelis
e
Menten kinetics. One can observe that the general shape of the curve is
the same, except a stretch of the curve along the substrate concentration axis.
Noncompetitive inhibitors are not substrate analogs. Inhibitors bind on sites other than the
active site and reduce enzyme affinity to the substrate. Noncompetitive enzyme inhibition
can be described as follows:
K
m
k
2
E
+
S
ES
E
+
+
P
+
I
+
I
(8.49)
K
I
K
I
K
m
EI + S
EIS
K
m
¼
½
E
½
S
¼
½
EI
½
S
(8.50)
½
ES
½
EIS
K
I
¼
½
E
½
I
¼
½
ES
½
I
(8.51)
½
EI
½
EIS
½
E
0
¼½
E
þ½
ES
þ½
EI
þ½
EIS
(8.52)
and
r
p
¼ k
2
½
ES
(8.53)




















Search WWH ::

Custom Search