Biomedical Engineering Reference
In-Depth Information
Kinetics of this type thus provides information about the existence of a covalent intermediate.
Often the finding of double-displacement (or “ping-pong”) kinetics is used as evidence for
the existence of this intermediate, but other confirming information should be sought. The
kinetic pattern of this type of mechanism is unique. Following the same technique we
used earlier, the rate expression can be derived as
½
E
0
½
A
½
B
k
Q
þ
k
P
r
P
¼
(8.39)
k
A
þ
k
Q
k
A
þk
Q
½
k
B
þ
k
P
k
B
þ k
P
½
½
A
½
B
þ
B
þ
A
8.2.4.2. Allosteric Enzymes
Some enzymes have more than one substrate-binding site. The binding of one substrate to
the enzyme facilitates binding of other substrate molecules. This behavior is known as allo-
stery or cooperative binding, and regulatory enzymes show this behavior. The rate expression
in this case is
n
r
max
½
S
r
P
¼
(8.40)
n
K
0
m
þ½
S
where n
1 indicates positive cooperativity.
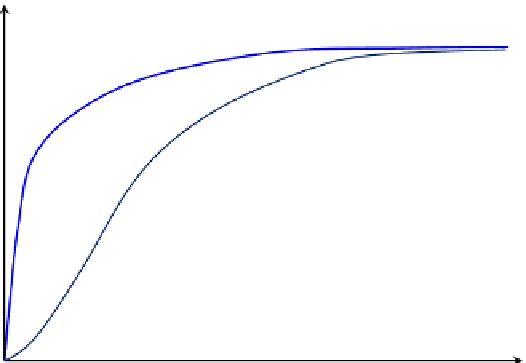
Figure 8.10
compares Michaelis
e
Menten kinetics with allosteric enzyme kinetics, indicating a sigmoidal
shape of r
P
~ [S] plot for allosteric enzymes. The shape of curve is different as the increase in
the reaction rate is slower at low substrate concentration due to effectively higher reaction
rate as expressed by Eqn
(8.16)
.
¼
cooperativity coefficient and n
>
8.2.4.3. Inhibited Enzyme kinetics
Certain compounds may bind to enzymes and reduce their activity. These compounds
are known to be enzyme inhibitors. Enzyme inhibitions may be irreversible or reversible.
r
max
Michaelis-Menten
r
P
Allosteric
1
2
r
max
0
[S] =
K
m
K
'
[S]
n
=
0
[S]
FIGURE 8.10
Comparison of Michaelis
e
Menten and allosteric enzyme kinetics.










Search WWH ::

Custom Search