Biomedical Engineering Reference
In-Depth Information
r
max
r
P
1
2
r
max
0
[S] =
K
m
0
[S]
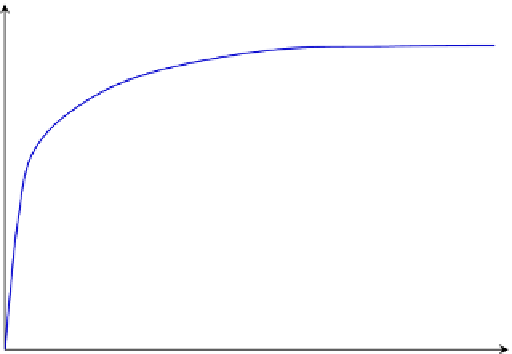
FIGURE 8.8
Effect of substrate concentration on the rate of product formation for an enzyme-catalyzed reaction.
Since the enzyme is not consumed, the conservation equation on the enzyme yields
½
E
¼½
E
0
½
ES
(8.5)
At this point, an assumption is required to simplify the rate expression eliminating the
requirement of concentrations other than the enzyme loading and substrate and product
concentrations.
8.2.2.1. The Fast Equilibrium Step Assumption
Henri and Michaelis and Menten used essentially this approach. Assuming the first step is
very fast, and equilibrium is reached when one considers the second step. That is to say
0 ¼ r
1
¼ k
1
½
E
½
S
K
1
½
ES
(8.6)
(Do you see why the rate is zero? This is contrary to what it states that this first step is very
fast.) Equation
(8.6)
yields
k
1
k
1
¼
½
E
½
S
K
m
¼
(8.7)
½
ES
Apply Eqn
(8.5)
if enzyme is conserved, then
¼
½
K
m
þ½
E
0
½
S
½
(8.8)
ES
S
Substituting Eqn
(8.8)
into Eqn
(8.3)
yields
k
2
½
E
0
½
S
K
m
þ½
r
max
½
S
r
P
¼ k
2
½
ES
¼
¼
(8.9)
K
m
þ½
S
S
where r
max
¼
k
2
[E]
0
.











Search WWH ::

Custom Search