Biomedical Engineering Reference
In-Depth Information
O
C
E Zn
2+
+ HCO
-
E Zn
2+
O
-
O
H
FIGURE 8.7
Metalloenzyme carbonic anhydrase facilitates the hydration of CO
2
to bicarbonate.
complex causes slight changes in the three-dimensional shape of the enzyme. This induced fit
of the substrate to the enzyme molecule may contribute to the catalytic activity of the
enzyme, too. The enzymes lysozyme and carboxypeptidase-A have been observed to change
their three-dimensional structure upon complexing with the substrate. Enzyme catalysis is
affected not only by the primary structure of enzymes but also by the secondary, tertiary,
and quaternary structures. The properties of the active site of enzymes and the folding char-
acteristics have a profound effect on the catalytic activity of enzymes. Certain enzymes
require coenzymes and cofactors for proper functioning.
Table 8.2
lists some enzymes and their cofactors and coenzymes.
8.2. ENZYME KINETICS
8.2.1. Introduction
A mathematical model of the kinetics of single substrate-enzyme-catalyzed reactions was
first developed by V. C. R. Henri in 1902 and by L. Michaelis and M. L Menten in 1913.
Kinetics of simple enzyme-catalyzed reactions are often referred to as Michaelis
e
Menten
kinetics or saturation kinetics. The qualitative features of enzyme kinetics are shown in
Fig. 8.8
, which is similar to Langmuir
e
Hinshelwood kinetics (Chapter 9). These models
are based on data from batch reactors with constant liquid volume in which the initial
substrate, [S]
0
, and enzyme, [E]
0
, concentrations are known. More complicated ES interac-
tions such as multisubstrate
e
multienzyme reactions can take place in biological systems.
An enzyme solution has a fixed number of active sites to which substrates can bind. At
high substrate concentrations, all these sites may be occupied by substrates or the enzyme
is saturated. Saturation kinetics can be obtained from a simple reaction scheme that involves a
reversible step for ES complex formation and a dissociation step of the ES complex.
k
1
k
2
E
E
þ
S
ES
/
þ
P
(8.2)
%
k
1
It is assumed that the ES complex is established rather rapidly, and the rate of the reverse
reaction of the second step is negligible. The assumption of an irreversible second reaction
often holds only when product accumulation is negligible at the beginning of the reaction.
Two major approaches used in developing a rate expression for the enzyme-catalyzed reac-
tions are (1) rapid-equilibrium approach and (2) pseudosteady-state approach.


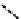















Search WWH ::

Custom Search