Biomedical Engineering Reference
In-Depth Information
enzyme is inhibited, tetanic shock and muscle paralysis follow. The enzyme is thus the target
for nerve gases and some insecticides. The enzyme has two subsites; one contains the nucle-
ophilic serine which is involved in the formation of an acetyl
e
enzyme intermediate (called
the esteratic site), and the other is negatively charged and provides a salt bridge to enhance
recognition and binding of the trimethylammonium region of acetylcholine. The serine acts
a nucleophile and attacks the ester linkage, presumably via the formation of a tetrahedral
intermediate. Then the choline is released, and an acyl enzyme intermediate is formed. Water
(OH) then releases the acetate from the acyl intermediate. The reaction mechanism is shown
in
Fig. 8.5
.
The enzyme is remarkably efficient; it has a turnover number (k
cat
, or the first-order reac-
tion rate constant) of 25,000 and thus cleaves one substrate molecule every 40
s. This rapid
rate of cleavage is crucial as nerve impulses can be carried at a rate of 1000 impulses/s, neces-
sitating the rapid removal of acetylcholine from the postsynaptic receptor.
Electrophilic catalysts, in contrast to nucleophilic catalysis, act by withdrawing electrons
from the reaction center of the intermediate and are thus electron sinks. They stabilize a nega-
tive charge. Examples of this mechanism involve coenzymes thiamine pyrophosphate and
pyridoxal phosphate. In many cases, including these coenzymes, electrophilic catalysis
involves the formation of Schiff bases. For example, acetoacetate decarboxylase catalyzes
the decarboxylation of acetoacetate to acetone and CO
2
. The mechanism involves the forma-
tion of a Schiff base involving a lysine residue. Acetoacetate decarboxylase participates in
the production of acetone by fermentation of sugars by anaerobic bacteria, such as
Clostridium acetobutylicum. This fermentation process was an important route to acetone
in World War I, when acetone was employed in the production of the explosive cordite
and chemical routes were not available. Other important Schiff base reactions include
aldolase and transaldolase reactions.
Acids and bases can catalyze reactions by either donating or accepting a proton which is
transferred in the transition state. When such a charged group develops in the transition
m
enzyme-substrate complex
acyl enzyme intermediate
O
C
O
CH
3
NH
3
O
C
H
3
C
+
O
+
OH
NH
2
OH
_
_
Ser
Ser
anionic site
cationic site
H
2
O
OH
+
CH
3
COOH
_
Ser
FIGURE 8.5
The mechanism of action of acetylcholine esterase.















































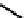





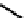









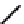


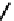


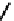

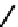






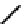

















Search WWH ::

Custom Search