Biomedical Engineering Reference
In-Depth Information
should note that this large change in rate for a relatively small change in activation energy is
due to the exponential dependence of rate on activation energy. In this case,
e
29;000
e
75;00029;000
8:314293
e
75;000
¼ 1:5910
8
if the preexponent factor
the ratio of the rates is
¼
8:314293
1:987293
remains the same.
The molecular aspects of enzyme
e
substrate (ES) interaction differ for different enzyme
and substrate pairs. Various studies using crystallography, X-ray, and Raman spectroscopy
have revealed the presence of the ES complex. The interaction between the enzyme and its
substrate is usually by weak forces. When the substrate enters the active site of an enzyme,
it will be held initially by noncovalent forces. These noncovalent forces responsible for
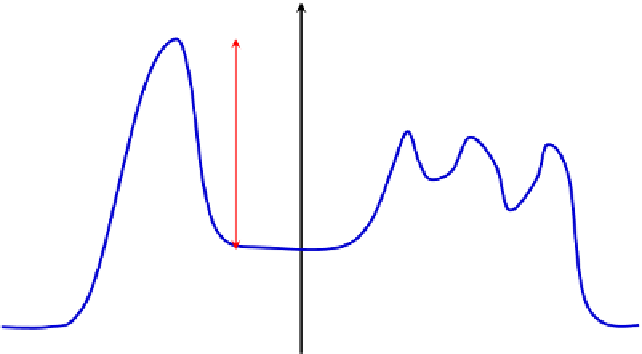
binding may be employed to lower the activation energy of the reaction as shown in
Fig. 8.3
. The types of noncovalent forces that are involved can be summarized as follows:
a) Electrostatic interactions include charge
e
charge (1/d
S-enz
, i.e. inversely proportional to the
distance between the charged enzyme active site to the charged substrate), dipole
e
dipole
ðd
6
ðd
4
ðd
6
interactions.
The magnitude of these forces depends on the distance between molecules, varying with
the distance (d
S-enz
) in the manner indicated above. All depend inversely on the dielectric
constant of the solvent between the ions or dipoles. b) van der Waals forces are comprised of
electron cloud repulsion
S
-
enz
Þ
, charge-induced dipole
S
-
enz
Þ
, and dipole-induced dipole
S
-
enz
Þ
ðd
12
ðd
6
.
The sum of these is described by the Lennard
e
Jones 6
e
12 potential. Dispersion forces are
not large, but in an enzyme, the sum of all such forces between substrate and enzyme may
be quite significant. c) Hydrogen bonds are important in biological systems and occur when
two electronegative atoms are bound to a common proton. Often oxygen is one of the atoms.
d) Hydrophobic forces reflect the tendency of apolar molecules to partition from an aqueous
S
-
enz
Þ
and attractive dispersion forces (London forces)
S
-
enz
Þ
No Enzyme Present (Uncatalyzed)
Enzyme Present
(Catalyzed)
E
U
E
C
Substrate
H
R
Product
Product
Reaction Progress
Reaction Progress
FIGURE 8.3
Activation energies of enzymatically catalyzed and uncatalyzed reactions. Note that E
U
<
E
C
.























Search WWH ::

Custom Search