Biomedical Engineering Reference
In-Depth Information
1
2
1
2
A
&
B
1
1
2
2
AB*
C
FIGURE 6.5
Analogy of chemical reaction pathway and electric circuit: reaction rates (r's) are interpreted the
same as fluxes (J's) moving from one node to the next.
which can be solved to give
k
1
C
AB
¼
C
C
(6.45)
A
B
k
1
þ
k
2
By virtue, we assume the concentration C
AB*
is negligibly small as comparedwith C
A
andC
B
.If
this is not the case, one needs to examine its effect on the concentrations of AandB (see footnote
from the previous section). Thus, the overall reaction rate, for reaction
(6.32)
, is given by
k
k
1
2
r
¼
r
C
¼
r
2
¼
k
C
AB
¼
C
A
C
B
(6.46)
2
k
1
þ
k
2
Therefore, we have derived at the rate expression using PSSH. This reaction rate expres-
sion is quite similar to that obtained from fast-equilibrium step (FES) approximation.
However, the reaction rate constant is different. When experiments are conducted to deter-
mine the rate expression, one can hardly distinguish
Eqn (6.46)
from
(6.43)
or
(6.42)
. There-
fore, both FES and PSSH are useful tools for kinetic or reaction rate analyses.
One advantage of PSSH is that if one draws the reaction system in analog with an electric
circuit as shown in
Fig. 6.5
, the reaction rate is like the electric current or flux. The flux is equal
throughout the electric circuit, passing any given nodes (or intermediates). There is no loss of
mass along the reaction pathway. This assumption is applicable and useful after the reaction
starts (some product has already been formed) and before the reaction ends (there are still
reactants available). This assumption is also less harsh than the FES approximation. There-
fore, PSSH is widely used in kinetic analyses.
6.4. UNIMOLECULAR REACTIONS
In this section, we are dealing with reactions:
(6.47)
which can be found in isomerization and decomposition reactions. In the stoichiometry, only
one molecule or one species (to be more precise) is acting as the reactant.
At first glance, it seems paradoxical to treat unimolecular reactions, in which a single
molecule is apparently involved in reaction, in terms of a collision theory based on pairwise
interactions. Indeed, we have developed a rather specific picture of a chemical reaction from
the hard-sphere collision model, in which the energetics of reaction is represented in terms of
relative kinetic energy.
A
/
products





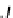






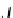

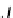




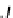
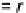

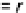










Search WWH ::

Custom Search