Biomedical Engineering Reference
In-Depth Information
on the discussions we have earlier, we know that in order for the reaction to occur, the
“molecular bundle” must overcome an energy barrier. That is, there is a switch for the reac-
tion to occur, unless it is the spontaneous decaying reaction like that of nuclear reaction.
Therefore, it is expected that not every collision would lead to a successful reaction.
In the new visualization, consider a collision between hard-sphere molecules A and B with
relative velocity v
r
and b as an impact parameter which can be related to, but is generally not
equal to, d
AB
. The general view corresponds to
Fig. 6.4
. Here
v
are velocity compo-
nents in the parallel and perpendicular directions to the line of motion. We can also write an
energy expression in terms of these velocity components
and
v
t
jj
M
1
E
r
¼
1
2
þ
M
v
jj
þ
v
2
t
Þ
ð
(6.22)
A
B
The second term of this expression is the energy directed along the line of centers and, in the
hard-sphere model, we assume that this is the component of energy involved in the reaction.
Let us call this energy along the line of centers E
c
. E
c
is a function of the distance between two
colliding (or interacting) spheres. From
Fig. 6.4
we have
v
2
t
v
jj
þ
E
c
E
r
¼
v
2
t
¼ 0;
b
>
d
AB
(6.23)
v
2
t
v
jj
þ
b
2
d
2
AB
E
c
E
r
¼
v
2
t
¼ 1
;
b
d
AB
Now, we will further assume that there is an energy-dependent reaction probability and, in
the most simple approach, that this is just an “on
e
off” switch such that the probability of
reaction is zero if E
c
<
E
a
and is unity if E
c
>
E
a
, where E
a
is the minimum energy for reaction.
If we write this out formally,
dð
E
c
Þ¼0;
E
c
<
E
a
(6.24)
dð
E
c
Þ¼1;
E
c
E
a
FIGURE 6.4
The reactive hard-sphere model.


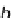
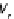
















Search WWH ::

Custom Search