Biomedical Engineering Reference
In-Depth Information
reactor, divided by the reactor volume. Thus, one can refer the left-hand side as the molar
supply rate per reactor volume or simply molar supply rate. That is
F
A
0
F
A
V
MS
A
¼
(5.54)
which can be rearranged to give
f
A
V
¼ Q
0
C
A
0
f
A
V
¼
C
A
0
f
A
s
MS
A
¼ F
A
0
(5.55a)
or
C
A
0
r
C
A
Q
0
C
A
0
QC
A
V
r
0
MS
A
¼
¼
(5.55b)
s
Therefore, the molar rate supply of A to a CSTR is linearly related to the conversion,
Eqn (5.55a)
. If the density remains constant,
r ¼ r
0
, then the molar supply rate of A to
a CSTR is also linearly related to the concentration of A. In other words, when density is
constant, the molar supply rate of A to the CSTR is a straight on the supply rate vs concen-
tration plane.
Let us now look at the right-hand side in the context of molar balance. It is the molar
consumption rate (or negative molar generation rate) of A. That is,
MC
A
¼r
A
(5.56)
The molar balance equation can thus be expressed as the molar supply rate of A to the reactor
(
MS
A
) equals to the molar consumption rate of A (
MC
A
) in the reactor. The solution to a CSTR
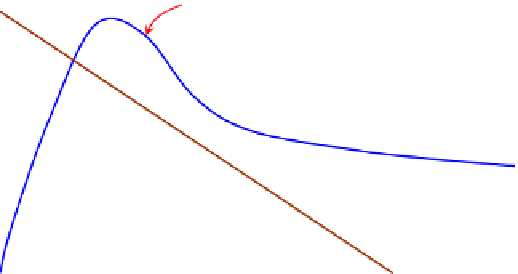
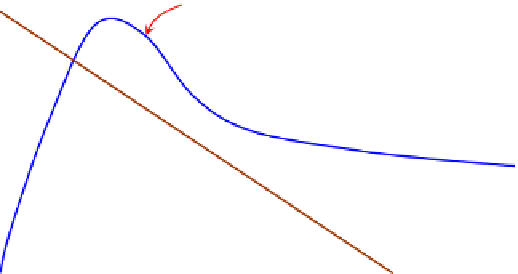
problem is thus visually illustrated in
Fig. 5.10
: on the two-dimensional graph of “molar
MC
A
or
-
r
A
MS
A
0
C
Ae
C
A0
0
C
A
FIGURE 5.10
Realizing mole balances in a CSTR. The molar consumption rate of A,
MC
A
, is same as the rate of
reaction of A,
r
A
, in the CSTR operating conditions, whereas the molar supply rate of A (molar feed rate of A
subtract the molar rate of A letting out of the CSTR),
MS
A
, changes linearly with the concentration for a constant
density reactor.













Search WWH ::

Custom Search