Biomedical Engineering Reference
In-Depth Information
H
2
O
CO
2
CH
4
0.1
H
2
0.01
0.001
CO
300
400
500
600
700
800
900
1000
1100
T
, °C
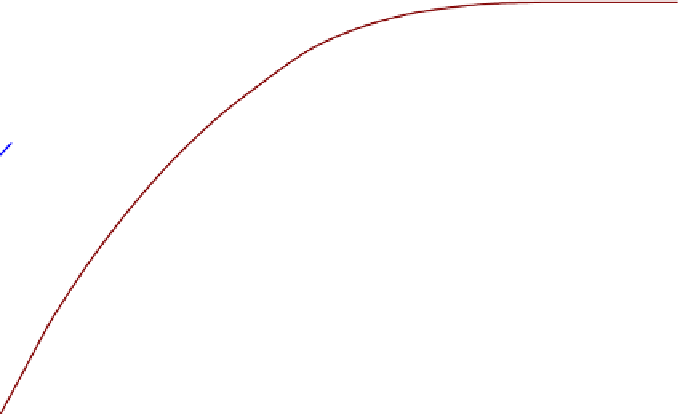
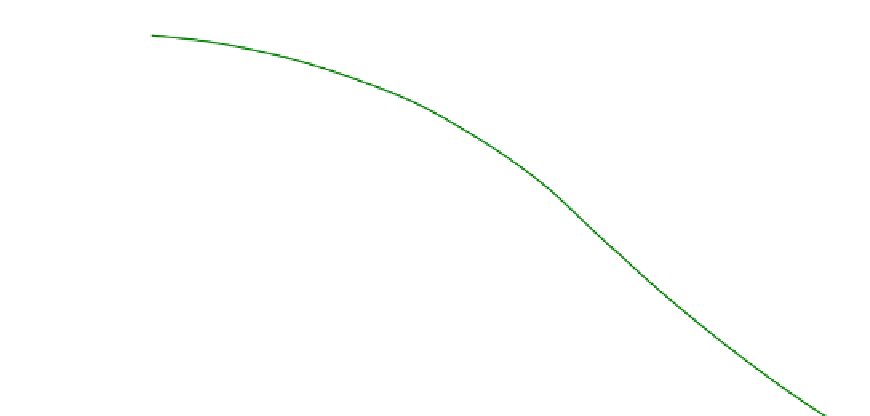
FIGURE 5.5
Equilibrium mole fractions for the carbon-steam reaction as a function of temperature.
Syngas can also be made using naphtha or other hydrocarbon feedstocks, such as the
methane from natural gas,
þ 3
H
2
(5.30)
Note that coal produces CO and H
2
in a 1:1 ratio, naphtha in a 1:2 ratio, and methane in
a 1:3 ratio. Because of the need in thermal energy to shift the reactions forward, oxygen
(or air) is usually supplied to partially oxidize the carbon (and hydrogen) to maintain
energy balance. An excess of H
2
CH
4
þ
H
2
O
/
CO
is thus usually desired; so alkanes are the preferred
feedstock.
Existing syngas plants operate by direct oxidation of natural gas
CH
4
þ
1
=
2
O
2
/
CO
þ 2
H
2
(5.31)
using pure O
2
from a liquid air plant. This process, called autothermal reforming, uses this
exothermic reaction in an adiabatic reactor and produces the 1:2 ratio of CO:H
2
that is ideal
for methanol or FT processes.
Today, more attention is paid to the gasification of renewable biomass. Lignocellulosic
biomass holds the most promising position in a sustainably renewable world. Dry lignocel-
lulosic biomass can be approximated by CH
1.47
O
0.57
. Gasification of lignocellulosic biomass
leads to
CH
1:43
O
0:57
þð0:43 þ 2n
o
2
Þ
H
2
O
n
o
2
O
2
/
CO
þð1:145 þ 2n
o
2
Þ
H
2
(5.32)






























































































































































































































































































































































Search WWH ::

Custom Search