Biomedical Engineering Reference
In-Depth Information
Reaction rate and concentration or activity. At this point, one may wonder if activity
should be used in the reaction rate expressions rather than the concentrations. Based on argu-
ments from nonequilibrium thermodynamics, Eqn
(3.52)
should be used to compute the reac-
tion rate. This suspicion is also founded due to the equilibrium constant relationships and the
reaction rate constants regulations arrived at thermodynamic equilibrium, Eqns
(3.78) and
(3.79)
. Thermodynamically, the chemical activity can be thought of as the effective concentra-
tion and therefore it can find uses in many places where (apparent) concentration appears.
However, for reaction rates, the chemical activity should not be used in place of concentration.
This conclusion is rather empirical than theoretical. (But again, nonequilibrium thermody-
namics is also empirical; one could alter the arguments for nonequilibrium thermodynamics
to fit the chemical kinetic behaviors.)
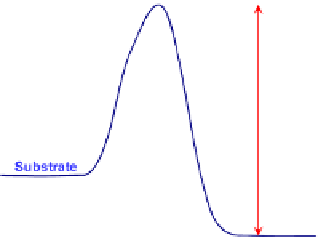
Figure 3.4
shows the variation of standard Gibbs free energy variation during a reaction. In
these illustrations, the concentration effects have been removed. Therefore,
Fig. 3.4
shows the
energetics of the reaction. Using arguments similar to nonequilibrium thermodynamics, Eqn
(3.52)
, and assuming that the chemical acitivity coefficient is independent of the concentra-
tions, one can readily arrive at that
!
D
S
f
!
DG
f
RT
E
f
RT
k
exp
k
f
¼
¼
k
exp
R
(3.90)
and
D
G
b
RT
S
b
R
D
E
b
RT
k
exp
k
b
¼
¼
k
exp
(3.91)
which forms the mathematical basis for the Transitional State Theory of chemical reaction
kinetics. To this point, we have established the relationship between properties of state
(enthalpy change, entropy change, and Gibbs free energy variation) with the reaction rate.
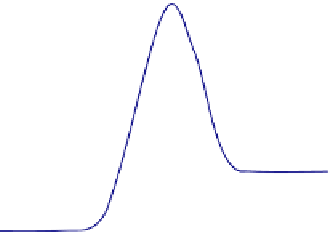
FIGURE 3.4
Plot of standard Gibbs free energy of reactants and products in a chemical reaction vs the reaction
coordinate. Although the overall (total) free energy always decreases after the reaction (
Fig. 3.2
), the standard free
energy can be increased or decreased. The reaction rate constants may be estimated via the standard Gibbs free
energy barriers. (a) Standard free energy variation for an energetic reaction. (b) Standard free energy variation for
an energy acquiring reaction.












































Search WWH ::

Custom Search