Biology Reference
In-Depth Information
Sequence analyses and secondary structure prediction revealed
that chorion proteins have a tripartite structure (Fig. 4.7), in that
they consist of three domains.
12,15
The central domain is conserved
in each of the two classes. The flanking N- and C-terminal domains
are more variable and contain characteristic tandem repeats.
12,15
A and B central domains show distant similarities suggesting that
the chorion genes constitute a superfamily derived from a single
ancestral gene.
14
The study of the properties of chorion proteins has long been
hampered by the fact that it has proven very difficult to purify
individual chorion proteins in large enough amounts of sufficient
purity for structural studies. Therefore, several chorion protein
peptide-analogues were synthesized and their structural and
assembly properties were studied under various conditions.
5,9,16-19
These studies, which are partly reviewed in this work, suggest that
silkmoth chorion is a natural protective amyloid.
4.2
Silkmoth Chorion Protein Peptide-Analogues
16
that can be
considered as a generic central domain of the A class of silkmoth
chorion proteins (Fig. 4.6). This peptide, referred to below as cA
peptide, is representative for about 20-30% of all the proteinaceous
Initially, a 51-residue peptide was synthesized,
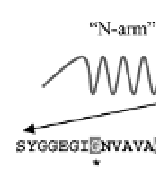
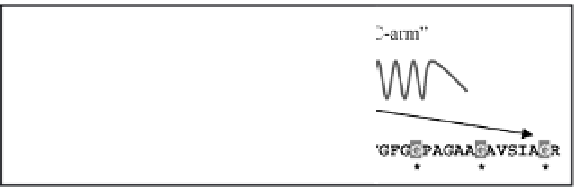
Figure 4.6
A schematic representation of the tripartite structure of
silkmoth chorion proteins of the A family. A highly conservative
central domain of invariant length, and two more variable
flanking “arms” constitute each protein. Characteristic,
tandemly repeating peptides are present both in the central
domain and in the “arms” (Hamodrakas, 1992, and references
therein). The amino acid sequence and relative position of
the synthetic cA peptide (one letter code), designed to be an
analogue of the entire central domain of the A family, is shown.
Invariant glycines (G), repeating every six residues, are boxed
and marked with an asterisk below the sequence.






Search WWH ::

Custom Search