Biology Reference
In-Depth Information
TheX-raydifractionpatternofasilkmothchorionshownin
Fig.4.4indicatesthat
β
-sheetisthedominantsecondarystructure
ofitsconstituentproteins.FT-Ramanspectroscopy(Fig.4.5)and
attenuatedtotalreflectanceFouriertransforminfrared(ATR-FTIR)
spectroscopy(Fig.4.6)suggestthatthe
β
12
-sheetsareantiparallel.
------- outer side
------- inner side
a
b
1100
1200
1300
1400
1500
1600
1700
1800
Wavenumber (cm
-1
)
FT-Raman(450-1800cm
-1
)spectrumof
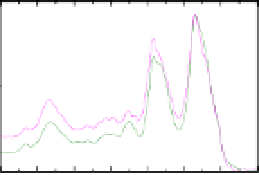
Figure 4.5
(a)
asilkmothchorion
of
Thesecondderivativespectrumisincluded.
Errorbarequalsto0.5
A. polyphemus.
σ
.ThepositionsoftheamideIat
-1
-1
1673cm
bandsintheRaman
spectrumarewellknownindicatorsof
andamideIIIat1235cm
-sheetsecondary
structureforsilkmothchorionproteins(Hamodrakas
β
et al.,
β
1982).The
-sheetsareveryuniformandverywellorganised
ascanbejudgedbytherathernarrowbandwidth(~30cm
-1
),
athalfmaximumintensity,oftheamideIbandat1673cm
-1
.
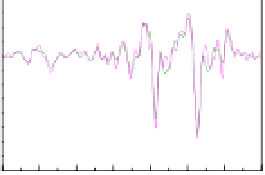
(b)ATR-FTIRspectratakenfromtheouter(magenta)and
inner(green)surfacesofan
silkmothchorion.
Thesecondderivativesareincluded.TheamideIandIIIbands
at1626and1230cm
-1
,respectively,clearlyindicatea
A. polyphemus
β
-sheet
typeofstructureforsilkmothchorionproteins.Theshoulderin
theamideIregion,at1694cm
-1
,suggeststhatthe
-sheetsare
anti-parallel.ReprintedfromBiopolymers(Biospectroscopy),
72,185-192,copyright(2003),withpermissionfromJohn
WileyandSons.SeealsoColourInsert.
β
About200proteinshavebeendetectedinthesilkmothchorion.
13
10
Theseproteinshavebeenclassifiedintotwomajorclasses,AandB.
Thegenefamiliesencodingtheseproteinsarerelatedandconstitute
asuperfamilywithtwobranches,the
14
α
-branchandthe
β
-branch.









































Search WWH ::

Custom Search