Biology Reference
In-Depth Information
longitudinal intermolecular assembly to result in amyloid-like
rodlets, there is another dimension of molecular recognition and
organization that drives lateral association of rodlets and orients
them with respect to the surface upon which assembly takes place.
On fungal structures, the hydrophobins assemble in a way that
presents the hydrophobic face to the outside of the aerial structure,
while the internal surface, adjacent to the cell wall, is hydrophilic.
10
A recent study of
spore germination has
demonstrated this with clarity, showing the disappearance in spore
surface hydrophobicity as germination occurs (Fig. 3.5).
Aspergillus fumigatus
37
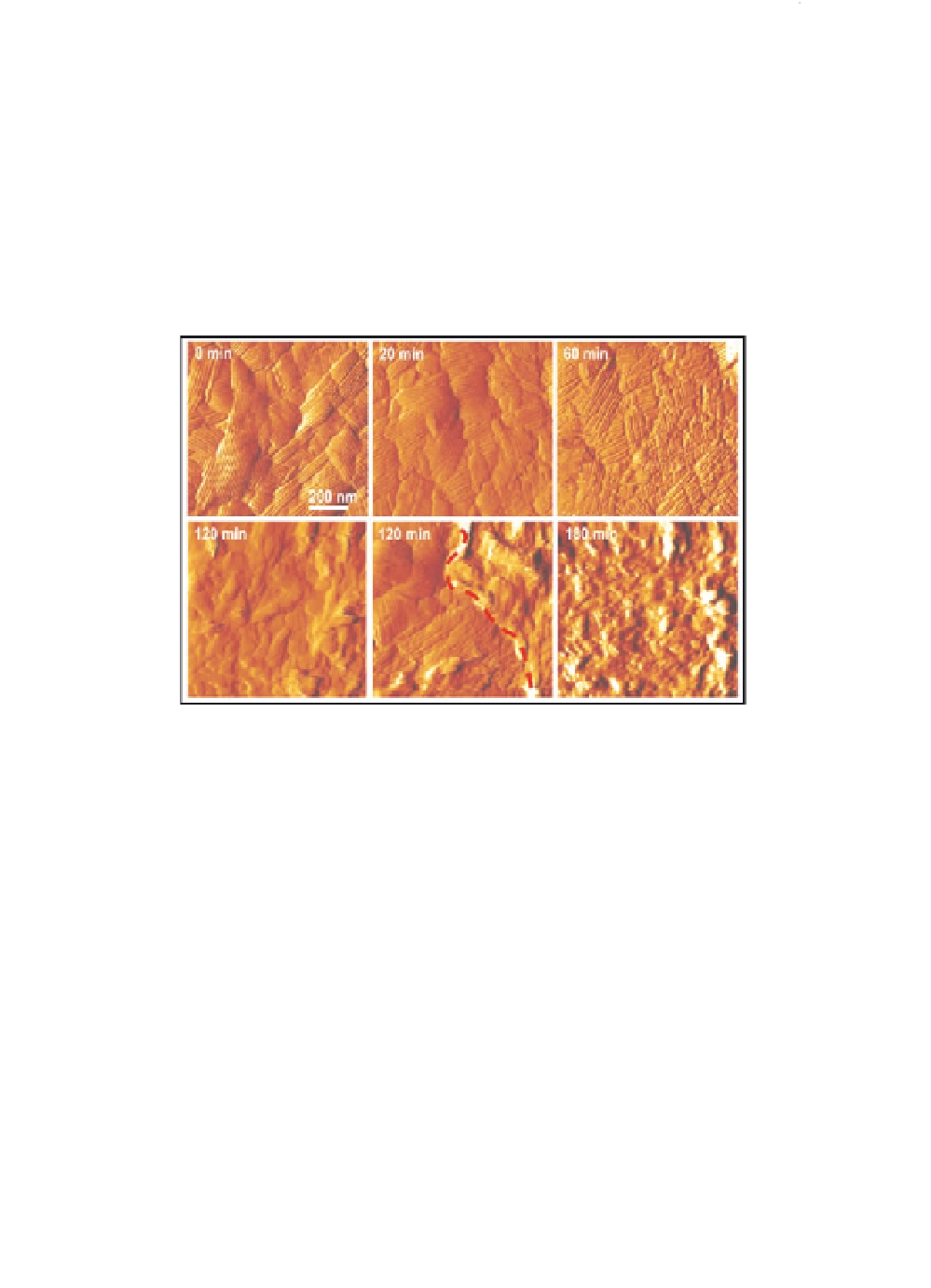
Figure 3.5
Structural dynamics of single germinating conidia. Series of
high-resolution AFM deflection images recorded on a single
spore during germination. Within 3 hours the crystalline rodlet
layer changes into a layer of amorphous material, presumably
reflecting inner cell wall polysaccharides. After two hours, both
rodlet and amorphous regions were found to coexist (lower
middle panel, left and right, respectively, of dashed line).
Figure reproduced with permission from Dague
et al
. (2007).
Copyright Biophysical Society. See also Colour Insert.
In contrast to the amyloid-like rodlets, the monolayers of
Class II hydrophobins are not fibrillar, or as robust, and do not bind
Thioflavin-T. Although they show some regular ordering, assemblies
of Class II hydrophobins do not display any reflections in either
X-ray scattering or diffraction patterns that could arise from
β
-sheet
38,39
structure.
Therefore, although both classes of hydrophobins form
amphipathic monolayers at surfaces, only the Class I hydrophobins
do so on the scaffold of an amyloid-like structure.
Search WWH ::

Custom Search