Biology Reference
In-Depth Information
or “fingerprints” is unproven. Such terms may only be valid for
synthesized or purified single component systems. The underlying
mechanism responsible for the strength of a natural adhesive can
thus be deduced from the characteristic mechanical properties
of the constituent molecules, rather than from the biochemical
identification of specific molecules present.
a
600
b
400
200
0
-200
0
500
1000
Extension (nm)
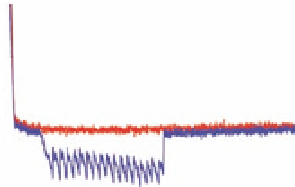
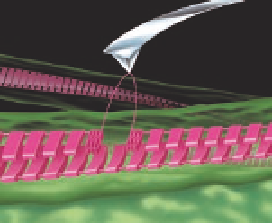
Figure 8.4
Nanoscale mechanical unfolding of amyloid fibril β-sheets in
algal adhesive. (a) A representative AFM force-extension curve
from holdfasts of the green alga
, showing highly
ordered sawtooth structures. (b) Schematic model of the
mechanical manipulation of a single intermolecular β-sheet
(pink) at the surface of an amyloid fibril (green) by an AFM tip.
The schematic shows interacting molecular building blocks of
intramolecular β-sheets running perpendicular to the direction
of the fibril making up extended intermolecular β-sheets that
form the fibril. The “hidden length” is contained within the
folds of each individual monomer (detail is shown only below
the tip) and the “sacrificial bonds” exist between these folds.
Interactions between the monomers must be significantly
stronger than these sacrificial bonds and imply cross-linking
between each individual protein, as indicated with the solid line
running along the back of the manipulated intermolecular β-
sheet. With kind permissio
n from Springer Science + Business
Media:
P
.
linearis
, Nanoscale mechanical
characterisation of amyloid fibrils discovered in a natural
adhesive,
Journal of Biological Physics
(2006) 393-401, A. S. Mostaert, M. J. Higgins, T.
Fukuma, F. Rindi, S. P. Jarvis, figures 2(a), 5.
14
See also Colour
Insert.
32
The repetitive sawtooth response can be quantitatively analysed
using the worm-like chain model for polymers
36,38
that provides,
amongst other parameters, a persistence length. In the case of
pulling apart the protein monomer constituents of an amyloid fibril,
the persistence length approximates the size of an amino acid (this

Search WWH ::

Custom Search