Biology Reference
In-Depth Information
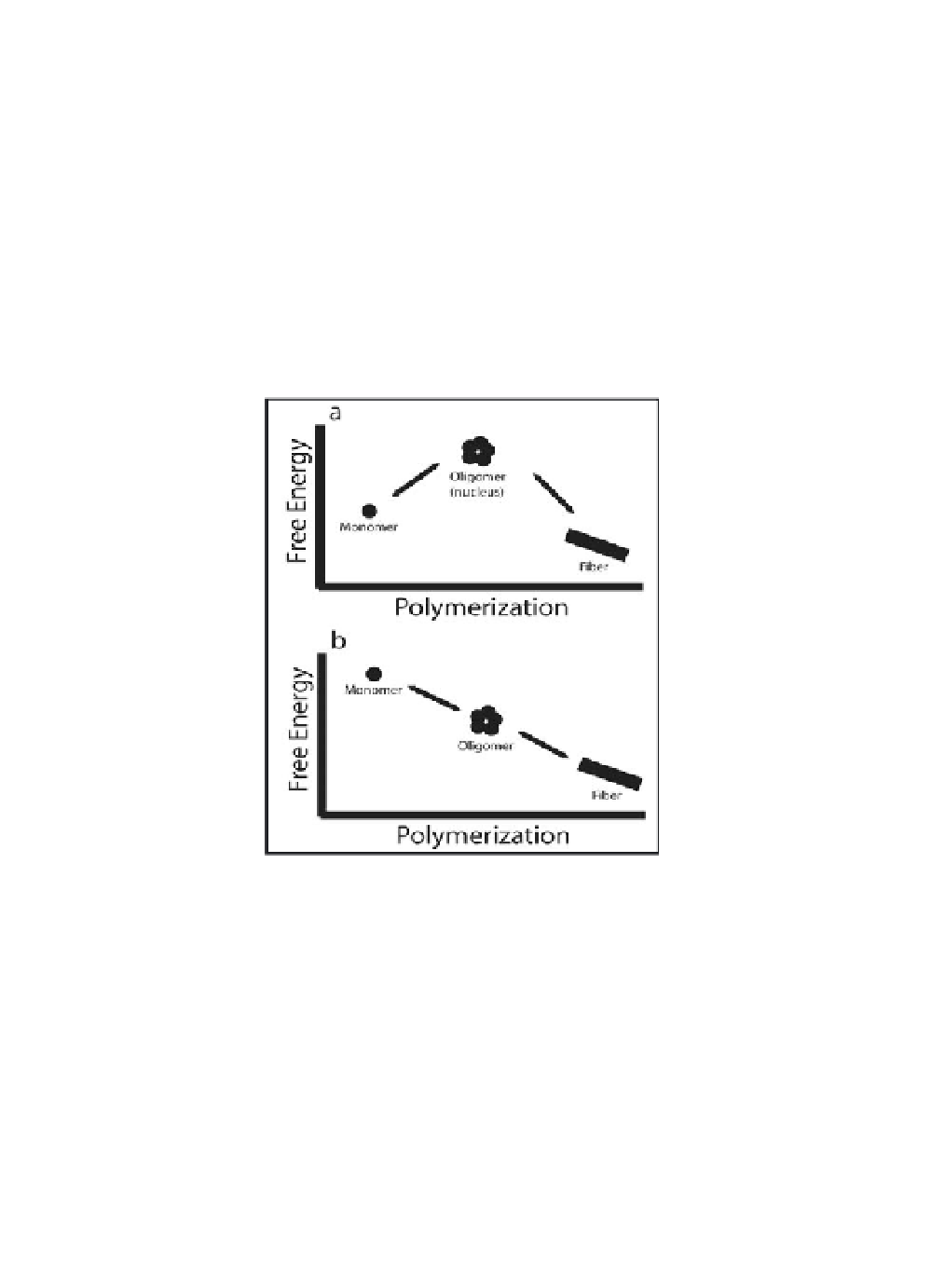
monomer addition events, including dimerizations, are energetically
favourable (Fig. 1.2b). Many additional mechanistic paradigms will
likely be discovered; one example is the recently described nucleated
conformational conversion mechanism.
11
Many, if not most, proteins can form amyloid under modestly
denaturing conditions
12
suggesting that amyloid is a widely
accessible, low-energy protein quaternary structure derived from
partly denatured states. Amino acid composition can bias a given
polypeptide towards amyloid formation. Hydrophobic sequences
with a paucity of charge are particularly amyloidogenic, although
the specific sequence determinants for amyloid formation remain
poorly understood.
13
Figure 1.2
Amyloid formation can occur by many mechanisms, two of
which are illustrated here. (a) In a nucleated polymerization,
monomer assembly into a high-energy oligomeric nucleus
is rate-limiting, with rapid polymerization to form fibres
after nucleus formation. (b) In a downhill polymerization,
all oligomerization steps are energetically favourable. The
biophysical characteristics of a polypeptide dictate, in part,
what mechanism will be observed. Conditions such as peptide
concentration and solution composition can also modulate the
mechanism. Our understanding of amyloidogenesis
is
poor; alternate mechanisms, influenced by chaperones and for
example, are likely to be found.
in vivo


Search WWH ::

Custom Search